Journal Description
Catalysts
Catalysts
is a peer-reviewed open access journal of catalysts and catalyzed reactions published monthly online by MDPI. The Romanian Catalysis Society (RCS) are partners of Catalysts journal and its members receive a discount on the article processing charge.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, SCIE (Web of Science), Inspec, CAPlus / SciFinder, CAB Abstracts, and other databases.
- Journal Rank: JCR - Q2 (Chemistry, Physical) / CiteScore - Q1 (General Environmental Science )
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 13.9 days after submission; acceptance to publication is undertaken in 2.6 days (median values for papers published in this journal in the second half of 2024).
- Recognition of Reviewers: reviewers who provide timely, thorough peer-review reports receive vouchers entitling them to a discount on the APC of their next publication in any MDPI journal, in appreciation of the work done.
Impact Factor:
3.8 (2023);
5-Year Impact Factor:
3.9 (2023)
Latest Articles
Photocatalytic Activity of Cu2O-Loaded TiO2 Heterojunction Composites for the Simultaneous Removal of Organic Pollutants and Bacteria in Indoor Air
Catalysts 2025, 15(4), 360; https://doi.org/10.3390/catal15040360 (registering DOI) - 6 Apr 2025
Abstract
This research investigates the enhanced photocatalytic activity of cuprous oxide (Cu2O) nanoparticles (NPs)-titanium dioxide (TiO2) nanotube (NT) composites for air purification, focusing on the removal of volatile organic compounds (VOCs) and Escherichia coli (E. coli) bacteria under
[...] Read more.
This research investigates the enhanced photocatalytic activity of cuprous oxide (Cu2O) nanoparticles (NPs)-titanium dioxide (TiO2) nanotube (NT) composites for air purification, focusing on the removal of volatile organic compounds (VOCs) and Escherichia coli (E. coli) bacteria under simulated sunny light. Cu2O-NPs were successfully deposited onto TiO2-NTs via the successive ionic layer adsorption and reaction method. The resulting p- and n-type semiconductor heterojunction nanocomposites were characterized using various techniques, including scanning electron microscopy, transmission electron microscopy, ultraviolet–visible-light spectroscopy, and chlorinated radicals. The photocatalytic activity was evaluated for different VOCs present in indoor air (butadione, chloroform, and butyraldehyde) in the presence of E. coli bacteria. The results showed that the Cu2O-NPs/TiO2-NTs composites exhibited enhanced photocatalytic activity compared to pure TiO2-NTs. The Langmuir–Hinshelwood model was used to describe the degradation kinetics, revealing that Cu2O loading and the nature of the target pollutant influence the photocatalytic efficiency. This study has also highlighted the role of chlorinated radicals in the degradation process, especially for chloroform. The degradation process of chloroform generated chlorine radicals, which not only contributed to the degradation of other VOCs, but also enhanced the overall oxidative capacity of the system. This synergistic effect was observed to accelerate pollutant removal and improve the antibacterial efficacy against E. coli. The Cu2O-NPs/TiO2-NTs composites demonstrated significant reusability and antibacterial properties, highlighting their potential for sustainable indoor air purification applications.
Full article
(This article belongs to the Section Photocatalysis)
►
Show Figures
Open AccessArticle
Activated Carbons as Supports for Sulfided Mo-Based Catalysts Intended for the Hydroprocessing of Lipidic Feedstocks
by
Antônio M. de Freitas Júnior, Ruana D. Brandão, Jeremie Garnier, Myller S. Tonhá, Wagner da N. Mussel, Daniel Ballesteros-Plata, Enrique Rodríguez-Castellón and Marcos J. Prauchner
Catalysts 2025, 15(4), 359; https://doi.org/10.3390/catal15040359 (registering DOI) - 6 Apr 2025
Abstract
The production of hydrocarbon-based biofuels has been the target of intense research worldwide. In this context, the core goal of the present work was to investigate the use of mesopore-rich activated carbons (ACs) as support for sulfided Mo-based catalysts intended for the hydroprocessing
[...] Read more.
The production of hydrocarbon-based biofuels has been the target of intense research worldwide. In this context, the core goal of the present work was to investigate the use of mesopore-rich activated carbons (ACs) as support for sulfided Mo-based catalysts intended for the hydroprocessing of lipidic feedstocks. The key motivations for the work were that, in comparison to traditional inorganic supports such as Al2O3, ACs are less propense to form coke, due to their lower acidity, and are highly resistant to hydrolysis, which is a very important aspect in the hydroprocessing of lipidic feedstocks because water is abundantly produced during the process. Furthermore, the porosity of ACs can be tailored to give rise to a high mesopore content, which is important for improving the access of bulky triglyceride molecules to metallic active sites located inside the pores network. A systematic study on the effects of the preparation conditions on the properties and performance of the obtained catalysts was carried out for the first time. The highest hydrodeoxygenation (HDO) activity was verified for the catalyst prepared through sequential deposition of Mo and Ni by wet impregnation. The prepared catalyst presented better performance for coconut oil HDO than an industrial sulfided NiMo/Al2O3 catalyst. Furthermore, it presented good stability, provided that the sulfidation degree was kept high. The obtained results evidenced that ACs have great potential to replace inorganic support in sulfided Mo-based catalysts.
Full article
(This article belongs to the Collection Nanocatalysis Towards Energy Transition and Environmental Sustainability)
►▼
Show Figures

Graphical abstract
Open AccessFeature PaperArticle
Understanding Lipase-Deep Eutectic Solvent Interactions Towards Biocatalytic Esterification
by
Can Liu and Jian Shi
Catalysts 2025, 15(4), 358; https://doi.org/10.3390/catal15040358 (registering DOI) - 6 Apr 2025
Abstract
Deep eutectic solvents (DESs) have shown promise as a medium for extracting polar volatile fatty acids (VFAs) and in situ esterification of the extracted molecules using lipases. This solvent enhanced biocatalysis process can potentially streamline VFA separation from fermentation broth by integrating conversion
[...] Read more.
Deep eutectic solvents (DESs) have shown promise as a medium for extracting polar volatile fatty acids (VFAs) and in situ esterification of the extracted molecules using lipases. This solvent enhanced biocatalysis process can potentially streamline VFA separation from fermentation broth by integrating conversion and extraction steps. Two commercial lipases from Aspergillus oryzae (AoL) and Candida rugosa (CrL) were evaluated in reaction systems containing hydrophilic or hydrophobic DESs using a newly optimized lipase assay. The optimal pH for both lipases was around 5.0, with a slight reduction in activity at pH 8.0 and a significant inhibition at pH 2.0. The impact of DES concentration on lipase activity varied depending on the specific DES–lipase pairs. Most hydrophilic DESs show good compatibility with the tested lipases. Specifically for choline chloride/ethylene glycol (1:2) and choline chloride/levulinic acid (1:2), taking into account the influence of pH, CrL activity increased with DES concentration. However, the hydrophobic DES thymol/2,6-dimethoxyphenol (1:2) demonstrated enhanced inhibitory effects on both lipases. Docking simulation helped explain the ligand–protein interactions but showed limited capability in predicting the compatibility of specific DES–lipase pairs due to its constraints in simulating flexible protein structures and the complex interactions between DES components and water.
Full article
(This article belongs to the Special Issue Topical Advisory Panel Members' Collection Series: Biomass Catalytic Conversion)
►▼
Show Figures

Graphical abstract
Open AccessArticle
Copper Molybdate-Catalyzed Esterification of Levulinic Acid: A Heterogeneous Approach for Biofuel Synthesis
by
Alyne Pereira de Oliveira Ribeiro, Wyvirlany Valente Lobo, Talles André Feitosa de Carvalho, José Milton Elias de Matos, Flávio Augusto de Freitas, Yurimiler Leyet Ruiz, Robert S. Matos, Ştefan Ţălu, Henrique Duarte da Fonseca Filho, Lianet Aguilera Domínguez, Walter Ricardo Brito and Francisco Xavier Nobre
Catalysts 2025, 15(4), 357; https://doi.org/10.3390/catal15040357 (registering DOI) - 6 Apr 2025
Abstract
The catalytic esterification of levulinic acid (LA) to methyl levulinate (ML) was investigated using copper molybdate (Cu3(MoO4)2(OH)2) as a heterogeneous catalyst. The catalyst, synthesized via chemical precipitation, exhibited a monoclinic structure with self-assembled nanoplates forming
[...] Read more.
The catalytic esterification of levulinic acid (LA) to methyl levulinate (ML) was investigated using copper molybdate (Cu3(MoO4)2(OH)2) as a heterogeneous catalyst. The catalyst, synthesized via chemical precipitation, exhibited a monoclinic structure with self-assembled nanoplates forming spherical mesostructures. Structural characterization confirmed its high crystallinity, while textural analysis revealed a BET surface area of 70.55 m2 g−1 with pore sizes in the nanometric range (1–6 nm). The catalytic performance was systematically evaluated under varying reaction conditions, including temperature, catalyst dosage, reaction time, methanol-to-LA molar ratio, alcohol type, and catalyst reusability. Optimal conversion of 99.3% was achieved at 100 °C, a 1:20 methanol-to-LA molar ratio, 5% catalyst loading, and a reaction time of 4 h. Comparative analysis with other heterogeneous catalysts demonstrated superior efficiency and stability of Cu3(MoO4)2(OH)2, with minimal activity loss over four reuse cycles (final conversion of 77.1%). Mechanistic insights suggest that its high activity is attributed to Lewis and Brønsted acid sites, facilitating efficient esterification. This study underscores the potential of copper molybdate as a sustainable and recyclable catalyst for biofuel additive synthesis, advancing green chemistry strategies for biomass valorization.
Full article
(This article belongs to the Special Issue Sustainable Catalysis for Green Chemistry and Energy Transition)
►▼
Show Figures

Graphical abstract
Open AccessArticle
Heterogeneous Acid Catalytic Filaments for Three-Dimensional Printing: Their Preparation, Characterization, and Reduction of Free Fatty Acids in Crude Palm Oil
by
Jarernporn Thawornprasert, Kritsakon Pongraktham and Krit Somnuk
Catalysts 2025, 15(4), 356; https://doi.org/10.3390/catal15040356 (registering DOI) - 5 Apr 2025
Abstract
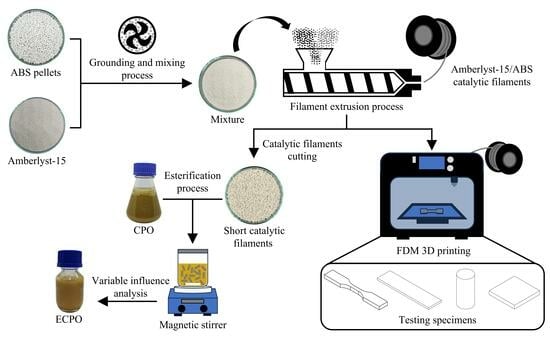
This study focuses on the fabrication and application of heterogeneous acid catalytic filaments for free fatty acid (FFA) reduction in crude palm oil (CPO) via esterification. Amberlyst-15 catalyst was blended with acrylonitrile butadiene styrene (ABS) using a single-screw filament extruder to produce Amberlyst-15/ABS
[...] Read more.
This study focuses on the fabrication and application of heterogeneous acid catalytic filaments for free fatty acid (FFA) reduction in crude palm oil (CPO) via esterification. Amberlyst-15 catalyst was blended with acrylonitrile butadiene styrene (ABS) using a single-screw filament extruder to produce Amberlyst-15/ABS catalytic filaments. A 5 wt.% concentration of fine Amberlyst-15 particles was considered optimal for blending with ABS, making them a suitable acid catalyst for FFA reduction. The mechanical properties, thermal behavior, and morphology of the Amberlyst-15/ABS catalytic filaments were assessed. The esterification process was optimized by varying three independent variables: the methanol-to-oil molar ratio, catalytic filament loading, and reaction time. The results revealed that under the recommended conditions—26.7:1 methanol-to-oil molar ratio, 78.5 wt.% catalytic filament loading, and a reaction time of 20.2 h at 500 rpm and 60 °C—the FFA content in CPO was reduced from 10.05 to 0.83 wt.%. Additionally, the reusability of the catalytic filaments was evaluated under the recommended conditions of the esterification process. The results demonstrated that the filaments remained effective for at least two cycles, achieving FFA levels below 2 wt.%, thereby confirming their stability and catalytic efficiency. The methodology employed in this study for the preparation and characterization of Amberlyst-15/ABS catalytic filaments offers a promising approach for fabricating acid catalytic materials via 3D printing, especially for heterogeneous catalysis in esterification reactions.
Full article
(This article belongs to the Section Industrial Catalysis)
►▼
Show Figures
Graphical abstract
Open AccessArticle
Ni-Doped Co-Based Metal–Organic Framework with Its Derived Material as an Efficient Electrocatalyst for Overall Water Splitting
by
Jingyuan Zhang, Hui Ni, Jianing Yu and Bin Zhao
Catalysts 2025, 15(4), 355; https://doi.org/10.3390/catal15040355 (registering DOI) - 5 Apr 2025
Abstract
Composite catalysts combining a metal–organic framework (MOF) with its derivatives have attracted significant attention in electrocatalysis due to their unique properties. In this study, we report the synthesis of a Ni-doped Co-1,4-benzenedicarboxylate (defined as Co3Ni1BDC) metal–organic framework via a
[...] Read more.
Composite catalysts combining a metal–organic framework (MOF) with its derivatives have attracted significant attention in electrocatalysis due to their unique properties. In this study, we report the synthesis of a Ni-doped Co-1,4-benzenedicarboxylate (defined as Co3Ni1BDC) metal–organic framework via a straightforward solvothermal method, aiming to enhance oxygen evolution reaction (OER) activity. The introduction of Ni modulated the electronic structure, yielding high catalytic activity with an overpotential (η100) of 300 mV and excellent stability for the OER. The Co3Ni1BDC material was further encapsulated with Co2P nanoparticles via a controlled phosphating annealing process, forming a hybrid electrocatalyst (Co3Ni1BDC@Co2P) to boost hydrogen evolution reaction (HER) performance. The Co3Ni1BDC@ Co2P catalysts exhibited superior HER performance with low overpotentials of η10 = 20 mV and η100 = 127 mV, outperforming the Co3Ni1BDC precursor. An alkaline electrolyzer assembled with Co3Ni1BDC//Co3Ni1BDC@Co2P achieved a cell voltage of 1.70 V at a current density of 20 mA cm−2. This work provides a valuable idea for designing efficient electrocatalysts for overall water splitting.
Full article
(This article belongs to the Special Issue Catalytic Activity of Two-Dimensional Materials and Their Heterostructures)
►▼
Show Figures

Graphical abstract
Open AccessArticle
Aluminum Microspheres Coated with Copper and Nickel Nanoparticles: Catalytic Activity in the Combustion of Ammonium Perchlorate
by
Yi Wang and Xiaolan Song
Catalysts 2025, 15(4), 354; https://doi.org/10.3390/catal15040354 (registering DOI) - 4 Apr 2025
Abstract
This study employed an in-situ displacement technique to eliminate the oxide layer present on the surface of micron aluminum (μAl). Utilizing the exposed metallic aluminum, we facilitated the displacement of copper and nickel nanoparticles. These nanoparticles, approximately 90 nanometers in size, were densely
[...] Read more.
This study employed an in-situ displacement technique to eliminate the oxide layer present on the surface of micron aluminum (μAl). Utilizing the exposed metallic aluminum, we facilitated the displacement of copper and nickel nanoparticles. These nanoparticles, approximately 90 nanometers in size, were densely adhered to the surface of the μAl particles. The elemental composition and structural characteristics of the composite particles were meticulously analyzed using Scanning Electron Microscopy (SEM), X-ray Diffraction (XRD), Energy Dispersive Spectroscopy (EDS), Vibrating Sample Magnetometry (VSM), and X-ray Photoelectron Spectroscopy (XPS). Subsequently, thermal analysis and combustion performance assessments were conducted to elucidate the catalytic effects of the composite particles ([nCu+nNi]/μAl) on the thermal decomposition and combustion efficiency of ammonium perchlorate (AP). The results elucidate that the nanoparticles immobilized on the surface of μAl are unequivocally metallic copper (nCu) and metallic nickel (nNi). Following the application of nCu and nNi, the oxidation reaction of μAl accelerated by nearly 400 °C; furthermore, the incorporation of [nCu+nNi]/μAl raised the thermal decomposition peak temperature of AP by approximately 130 °C. Notably, the thermal decomposition activation energy of raw AP reached as high as 241.7 kJ/mol; however, upon doping with [nCu+nNi]/μAl, this activation energy significantly diminished to 161.4 kJ/mol. The findings of the combustion experiments revealed that both the raw AP and the AP modified solely with μAl were impervious to ignition via the hot wire method. In contrast, the AP doped with [nCu+nNi]/μAl demonstrated pronounced combustion characteristics, achieving an impressive peak flame temperature of 1851 °C. These results substantiate that the nCu and nNi, when deposited on the surface of μAl, not only facilitate the oxidation and combustion of μAl but also significantly enhance the thermal decomposition and combustion dynamics of ammonium perchlorate. Consequently, the [nCu+nNi]/μAl composite shows considerable promise for application in high-burn-rate hydroxyl-terminated polybutadiene (HTPB) propellants.
Full article
(This article belongs to the Collection Nanotechnology in Catalysis)
Open AccessArticle
Effect of Mn/Cu Molar Ratios on CO Oxidation Activity of Mn-Cu Bimetallic Catalysts
by
Cong Liang, Yingchun Sun, Peiyuan Li, Ye Jiang, Xin Sun and Zhengda Yang
Catalysts 2025, 15(4), 353; https://doi.org/10.3390/catal15040353 - 4 Apr 2025
Abstract
The steel manufacturing industry is a major source of global air pollution, with sintering processes contributing over 70% of emissions, primarily carbon monoxide (CO), a significant uncontrolled pollutant. This study explores Mn-Cu bimetallic catalysts as a cost-effective and environmentally friendly alternative to noble
[...] Read more.
The steel manufacturing industry is a major source of global air pollution, with sintering processes contributing over 70% of emissions, primarily carbon monoxide (CO), a significant uncontrolled pollutant. This study explores Mn-Cu bimetallic catalysts as a cost-effective and environmentally friendly alternative to noble metal-based systems, addressing the urgent need for efficient CO oxidation catalysts. Mn-Cu catalysts with different Mn/Cu molar ratios were synthesized via hydrothermal methods and systematically characterized using XRD, XPS, BET, H2-TPR, etc., to assess their physicochemical properties and catalytic performance. The Mn4Cu1 catalyst demonstrated the highest CO oxidation activity, achieving complete conversion at 175 °C. This performance is attributed to its optimal Mn/Cu molar ratio, high specific surface area, abundant oxygen vacancies, and superior redox properties. The catalyst’s enhanced performance is further supported by its low reduction temperature and high Mn3+ and Cu+ content, which promote efficient electron transfer and oxygen activation. These findings highlight the crucial role of Mn/Cu molar ratios in optimizing catalytic performance and offer valuable insights for designing high-efficiency, low-cost catalysts to reduce CO emissions in industrial applications.
Full article
(This article belongs to the Special Issue Advanced Catalysts in Environmental Purification)
►▼
Show Figures

Graphical abstract
Open AccessCommunication
Computational Search for a Novel Effective Ligand for Ni-Catalyzed Asymmetric Hydrogenation
by
Evgeny V. Pospelov, Ivan S. Golovanov, Jianzhong Chen, Wanbin Zhang and Ilya D. Gridnev
Catalysts 2025, 15(4), 352; https://doi.org/10.3390/catal15040352 - 3 Apr 2025
Abstract
Using the DFT method, an analogue of R,R-t-Bu-BenzP* was tried as a potential ligand for Ni-catalyzed asymmetric hydrogenation. This ligand contains benzyl groups instead of the t-Bu groups in R,R-t-Bu-BenzP*. Computational results imply that the R
[...] Read more.
Using the DFT method, an analogue of R,R-t-Bu-BenzP* was tried as a potential ligand for Ni-catalyzed asymmetric hydrogenation. This ligand contains benzyl groups instead of the t-Bu groups in R,R-t-Bu-BenzP*. Computational results imply that the R,R-Benz-BenzP* ligand (1) is expected to provide excellent enantioselectivity in the Ni-catalyzed asymmetric hydrogenation of 1-phenylethanone oxime. The computed effectiveness of the R,R-Benz-BenzP* ligand is stipulated by its conformational flexibility, which helps stabilize the crucial transition states via a non-bonding interaction between the substrate and the catalyst. R,R-Benz-BenzP* ligands with CN- and OMe-substituted benzyl rings were also computed to possess the same effectiveness.
Full article
(This article belongs to the Section Computational Catalysis)
Open AccessArticle
Experimental Design and Numerical Optimization of Photochemical Oxidation Removal of Tetracycline from Water Using Fe3O4-Supported Fruit Waste Activated Carbon
by
Manasik M. Nour, Maha A. Tony, Hossam A. Nabwey and Shaaban M. Shaaban
Catalysts 2025, 15(4), 351; https://doi.org/10.3390/catal15040351 - 3 Apr 2025
Abstract
The ever-increasing importance of sustainable environmental remediation calls for academics’ contribution to satisfy such a need. The 3R’s criteria of recover, recycle and reuse is designed to sustain the waste stream to produce a valuable product. In this regard, the circular economy looks
[...] Read more.
The ever-increasing importance of sustainable environmental remediation calls for academics’ contribution to satisfy such a need. The 3R’s criteria of recover, recycle and reuse is designed to sustain the waste stream to produce a valuable product. In this regard, the circular economy looks to deliver banana peel waste as a photocatalyst for pharmaceutical effluent oxidation, which we investigated in this study. Banana peel waste is treated thermally and chemically then augmented with magnetite nanoparticles and labeled as ACBP-Fe3O4. The mixture is characterized through Scanning Electron Microscopy (SEM) and the composition of the composite material is attained by energy dispersive X-ray spectroscopy (EDX), and then introduced as a Fenton catalyst. The notable oxidation of tetracycline (TC), evaluated by TC removal and chemical Oxygen Demand (COD) oxidation tenancy, is achieved. The effectiveness of the operational parameters is also assessed and the most influenced parameters are optimized through numerical optimization based on a Response Surface Methodology (RSM) tool. The effects of initial pH value, ACBP-Fe3O4 and H2O2 concentrations on the oxidation efficiency of the Tetracycline were optimized at pH 6.6 and 350 mg/L and 43 g/L for H2O2 and ACBP-Fe3O4, respectively. Thermodynamics and kinetics were also studied and the experimental and model data revealed the reaction is spontaneous and exothermic in nature and follows the first-order reaction kinetics. Also, the thermodynamic results the reaction proceeds at a low energy barrier of 34.33 kJ mol−1. Such a system introduces the role of engineers and academics for a sustainable world without a waste stream.
Full article
(This article belongs to the Special Issue Remediation of Natural Waters by Photocatalysis)
►▼
Show Figures

Graphical abstract
Open AccessArticle
Fabricating Oxygen Vacancy-Rich Bi2WO6/Bi2S3 Z-Scheme Nano-Heterojunction on Carbon Fiber with Polydopamine for Enhanced Photocatalytic Activity
by
Jiantao Niu, Jiaqi Pan, Jianfeng Qiu and Chaorong Li
Catalysts 2025, 15(4), 350; https://doi.org/10.3390/catal15040350 - 2 Apr 2025
Abstract
The use of fibers or fabrics as frameworks for loading photocatalysts is beneficial in solving the problems of photocatalytic nanomaterials, which tend to agglomerate and are difficult to recycle. In this study, Bi2WO6/CFb and Bi2WO6/Bi
[...] Read more.
The use of fibers or fabrics as frameworks for loading photocatalysts is beneficial in solving the problems of photocatalytic nanomaterials, which tend to agglomerate and are difficult to recycle. In this study, Bi2WO6/CFb and Bi2WO6/Bi2S3/CFb photocatalytic fibers rich in oxygen vacancies were prepared using carbon fibers as the framework by the crystal seed attachment method and in situ growth method by using the self-polymerization and strong adhesion properties of dopamine. The results of SEM, TEM and XRD tests showed that Bi2WO6 and Bi2WO6/Bi2S3 nanosheets were uniformly and completely encapsulated on the surface of the carbon fibers. The results of XPS and EPR tests showed that Bi2WO6 nanosheets were rich in oxygen vacancies. The PL, transient photocurrent responses and EIS results showed that the introduction of Bi2S3 significantly improved the migration efficiency of the photogenerated carriers of Bi2WO6/Bi2S3/CFb, which effectively hindered the recombination of photogenerated electron–hole pairs. By conducting degradation experiments on p-nitrophenol and analyzing the bandgap structure, it was postulated that the heterojunction structure of Bi2WO6/Bi2S3/CFb in the Bi2WO6/Bi2S3 material was not Type-II but Z-scheme. As analyzed by the active species assay, the active species that played a major role in the degradation process were O2− and h+. The incorporation of a small amount of Bi2S3 resulted in enhanced photocatalytic degradation activity of Bi2WO6/Bi2S3/CFb toward tetracycline hydrochloride compared to Bi2WO6/CFb. The excellent photocatalytic performance of Bi2WO6/Bi2S3/CFb photocatalytic fibers can be attributed to the rapid transmission and separation performance and the high oxidation and reduction capacities of photogenerated electron–hole pairs formed by direct Z-scheme heterojunctions.
Full article
(This article belongs to the Section Catalytic Materials)
►▼
Show Figures
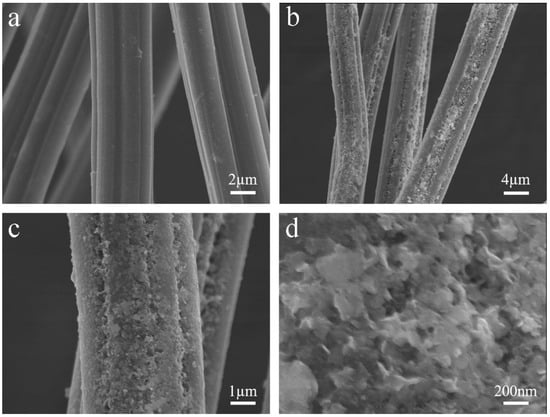
Figure 1
Open AccessFeature PaperArticle
Synthesis of Black g-C3N4 and Exploration of the Mechanism Underlying the Enhancement of Photocatalytic CO2 Reduction
by
Shaokun Lv, Jun Zhang, Xiaoke Chen, Yue Zou, Qiuli Chen, Yongsheng Yan and Pengxin Li
Catalysts 2025, 15(4), 349; https://doi.org/10.3390/catal15040349 - 2 Apr 2025
Abstract
The use of solar energy to convert CO2 into value-added chemicals is a promising sustainable development strategy. In this study, a black graphitic carbon nitride (CN-B) photocatalyst was fabricated through a single-step calcination process, employing phloxine B and urea as the precursor
[...] Read more.
The use of solar energy to convert CO2 into value-added chemicals is a promising sustainable development strategy. In this study, a black graphitic carbon nitride (CN-B) photocatalyst was fabricated through a single-step calcination process, employing phloxine B and urea as the precursor materials. The catalysts were characterized using TEM, XRD, FTIR, XPS and so on. The amount of prepolymer phloxine B was 25 mg, 35 mg and 45 mg, respectively, and the obtained samples were CN-B-0.025, CN-B-0.035 and CN-B-0.045. All samples were used for visible-catalyzed CO2 reduction. The experimental findings indicate that the CO evolution rate of the optimal photocatalyst CN-B-0.035 reaches 27.56 μmol gcat.−1 h−1. This value is nine-fold higher than that of pure CN, which has a CO evolution rate of 3.22 μmol gcat.−1 h−1. The excellent photocatalytic reduction performance is due to the following factors: Firstly, the exceedingly thin nanosheet structure of the catalyst enhances the velocity of the charge transfer, and transmission electron microscopy (TEM) analysis shows that the nanosheet thickness of the catalyst CN-B is significantly thinner. Secondly, the light absorption capacity of the catalyst is enhanced. The absorbance of CN-B increases significantly in the ultraviolet region and extends to the near-infrared region, as shown with UV diffuse reflection spectroscopy. Finally, the photothermal effect of CN-B causes the catalyst temperature to rise rapidly from 20 °C to 131 °C within 120 s, which further promotes photogenerated carrier separation. This research offers a novel approach to the development of photocatalysts aimed at the photothermal-assisted photocatalytic conversion of CO2.
Full article
(This article belongs to the Special Issue Mineral-Based Composite Catalytic Materials)
►▼
Show Figures
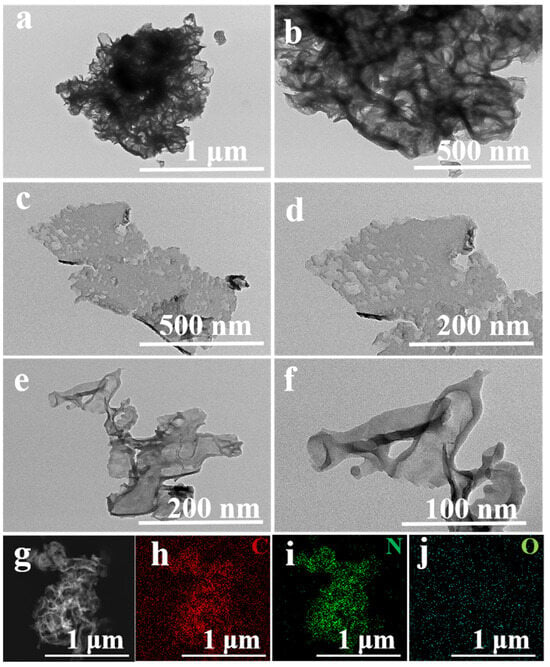
Figure 1
Open AccessFeature PaperArticle
Selective Conversion of Glycerol to Acetol: Effect of the Preparation Method of CuAl Catalysts and Reaction Phase
by
Francisco Maldonado-Martín, Lucía García, Joaquín Ruiz, Miriam Oliva and Jesús Arauzo
Catalysts 2025, 15(4), 348; https://doi.org/10.3390/catal15040348 - 2 Apr 2025
Abstract
A group of CuAl catalysts were synthesized with a Cu/Al molar ratio of 1:1 using different preparation methods: coprecipitation, surfactant assisted coprecipitation, polymeric precursor, and self-combustion and then screened for the selective dehydration of glycerol to acetol. The catalysts were employed in glycerol
[...] Read more.
A group of CuAl catalysts were synthesized with a Cu/Al molar ratio of 1:1 using different preparation methods: coprecipitation, surfactant assisted coprecipitation, polymeric precursor, and self-combustion and then screened for the selective dehydration of glycerol to acetol. The catalysts were employed in glycerol conversion at the same temperature (227 °C) in two different laboratory-scale systems, the first one at atmospheric pressure (gas phase) and the second one in a pressurized system at 34 absolute bar (liquid phase). The preparation method of the CuAl catalysts influenced the carbon yield to liquids and acetol selectivity. However, the reaction phase had a greater influence than the preparation method of the catalyst. In the gas phase, the carbon yield to liquids reached values above 40% and the carbon selectivity to acetol was higher than 90%. The highest acetol yield, 462.6 mgacetol/gglycerol, was obtained with the CuAl catalyst prepared by the surfactant-assisted coprecipitation method. This study provides a new perspective on catalyst design by highlighting the crucial role of preparation techniques in determining CuAl catalyst performance in the liquid and gas phases.
Full article
(This article belongs to the Special Issue Topical Advisory Panel Members' Collection Series: Biomass Catalytic Conversion)
►▼
Show Figures
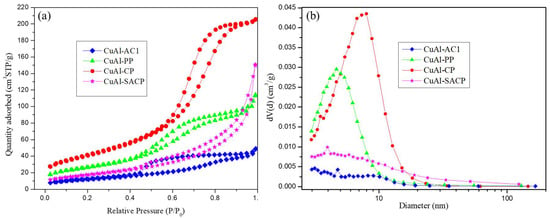
Figure 1
Open AccessArticle
Correlation Between the Apparent Rate Constant and the Dye Concentration—Effect of the Bottom Reflection Ability on the Photocatalytic Reaction Rate
by
Emil Lilov, Svetlozar Nedev, Vanya Lilova, Christian Girginov and Stephan Kozhukharov
Catalysts 2025, 15(4), 347; https://doi.org/10.3390/catal15040347 - 2 Apr 2025
Abstract
The dependence of the reaction rate on the solution layer thickness discovered in a previous work could be a powerful tool for investigating photocatalytic reactions. A reduction of the apparent rate constant with the growth of the dye concentration was found using this
[...] Read more.
The dependence of the reaction rate on the solution layer thickness discovered in a previous work could be a powerful tool for investigating photocatalytic reactions. A reduction of the apparent rate constant with the growth of the dye concentration was found using this dependence. This decrement follows a hyperbolic law. This dependence is explained based on the observed increment of the solution conductivity. In addition, it is confirmed experimentally that the reaction rate decreases with the solution depth growth. The possibility of independent determination of the reaction rate constant and the adsorption equilibrium constant has been discussed. Additionally, it is demonstrated that the vessel’s reflective bottom could increase the chemical reaction rate. The reason why other authors have not yet reported this effect is also discussed.
Full article
(This article belongs to the Section Photocatalysis)
►▼
Show Figures

Figure 1
Open AccessArticle
Frequency-Resolved Modulation Excitation Spectroscopy Methodology for Identifying Surface Reaction Species in Ethanol Oxidation on Gold Catalysts
by
Bhagyesha S. Patil, Alejandra Torres-Velasco and Juan J. Bravo-Suárez
Catalysts 2025, 15(4), 346; https://doi.org/10.3390/catal15040346 - 1 Apr 2025
Abstract
This study used in situ modulation excitation spectroscopy (MES) with varying frequencies in a single experiment to identify surface species during ethanol oxidation on Au/SiO2, Au/TiO2, Au/ZnO, and Au/SrTiO3. Fixed-bed reactor (FBR) tests (1 kPa ethanol, 1.5
[...] Read more.
This study used in situ modulation excitation spectroscopy (MES) with varying frequencies in a single experiment to identify surface species during ethanol oxidation on Au/SiO2, Au/TiO2, Au/ZnO, and Au/SrTiO3. Fixed-bed reactor (FBR) tests (1 kPa ethanol, 1.5 kPa O2, 513 K) showed that Au/SiO2 and Au/SrTiO3 had higher ethanol conversions. Au/SiO2 favored acetaldehyde, while Au/SrTiO3 yielded more acetates (acetic acid and ethyl acetate). Operando modulation excitation (ME)–phase sensitive detection (PSD)–DRIFTS, with ethanol and oxygen modulation, identified surface ethanol, acetaldehyde, acetates, ethoxy, and hydroxyl species. Oxygen modulation showed charge transfer to supports in Au/TiO2 and Au/ZnO. At the fundamental frequency (f0 = 1/90 Hz), ME–PSD–DRIFTS showed minimal adsorbed ethanol on Au/SiO2, indicating high ethanol conversion. Au/SrTiO3 had higher acetaldehyde consumption, correlating with increased acetates, consistent with FBR results. ME–PSD–DRIFTS at lower frequencies (0.07f0, 0.5 f0) and higher harmonics (2f0, 3f0) showed rapid ethoxy formation/decomposition, and slower acetaldehyde reactions, confirming acetaldehyde as a primary product and acetates as secondary products. Oxygen modulation revealed rapid hydrogen spillover and hydroxyl changes. Overall, operando spectroscopy via mass spectrometry confirmed the FBR findings.
Full article
(This article belongs to the Special Issue Spectroscopy in Modern Materials Science and Catalysis)
►▼
Show Figures
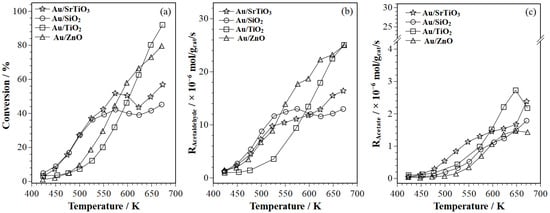
Figure 1
Open AccessArticle
Regulating the Structures of Carbon Cloth and Carbon Nanotubes to Boost the Positive Electrode Reaction of Vanadium Redox Flow Batteries
by
Xinyu Huang, Chuanyu Sun, Shuqi Liu, Bangsen Zhao, Mingming Ge and Huan Zhang
Catalysts 2025, 15(4), 345; https://doi.org/10.3390/catal15040345 - 1 Apr 2025
Abstract
Considering the various morphologies of carbon nanotubes (CNTs), it is expected to solve the contradiction between concentration polarization and electrochemical polarization in vanadium redox flow batteries (VRFBs). This paper investigates the structural evolution of CNTs grown on the surface of thermally oxidized carbon
[...] Read more.
Considering the various morphologies of carbon nanotubes (CNTs), it is expected to solve the contradiction between concentration polarization and electrochemical polarization in vanadium redox flow batteries (VRFBs). This paper investigates the structural evolution of CNTs grown on the surface of thermally oxidized carbon cloth (TCC) and their impact on the performance of VRFBs. The morphological results indicate that thermal oxidation treatment forms pores on the surface of the TCC, providing nucleation sites for CNT growth. Spiral-shaped CNTs (TCC@s-CNTs) were formed in a short growth time (1 h), and their high defect density originated from the non-steady-state supply of carbon sources and the dynamic behavior of the catalyst. While 3 h of growth forms a network structure (TCC@n-CNT), the van der Waals force drives the self-assembly of its three-dimensional network. Although the TCC@s-CNT exhibits high catalytic activity due to its high defect density and edge active sites, the performance of VRFBs is more dependent on the three-dimensional conductive network of the TCC@n-CNT. At 240 mA/cm2, the energy efficiency (EE) of a VRFB assembled with the TCC@n-CNT reaches 71%, and the capacity retention rate is 15% higher than that of the TCC@s-CNT. This work reveals the synergistic mechanism of CNT morphology regulation on electrode performance and provides theoretical guidance for the design of VRFB electrodes.
Full article
(This article belongs to the Section Catalysis for Sustainable Energy)
►▼
Show Figures
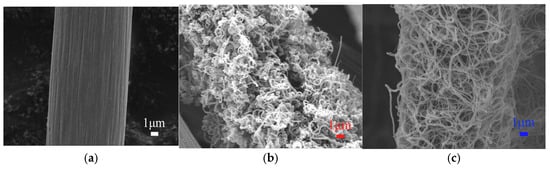
Figure 1
Open AccessFeature PaperArticle
Nitrone or Oxaziridine? Further Insights into the Selectivity of Imine Oxidation Catalyzed by Methyltrioxorhenium
by
Camilla Matassini, Marco Bonanni, Francesca Cardona and Andrea Goti
Catalysts 2025, 15(4), 344; https://doi.org/10.3390/catal15040344 - 1 Apr 2025
Abstract
The oxidation of imines may give several products, such as oxaziridines, nitrones, amides, and other rearranged compounds. Therefore, its selectivity is a challenge that various methods have to face. The controversial selectivity of the oxidation of imines using urea hydrogen peroxide (UHP) catalyzed
[...] Read more.
The oxidation of imines may give several products, such as oxaziridines, nitrones, amides, and other rearranged compounds. Therefore, its selectivity is a challenge that various methods have to face. The controversial selectivity of the oxidation of imines using urea hydrogen peroxide (UHP) catalyzed by methyltrioxorhenium (MTO) is addressed by varying the solvent, temperature, reaction time, amount of oxidant, and catalyst used. The reactivity and selectivity of the oxidation of imines proved to be particularly sensitive to the type of solvent. The use of methanol furnished the corresponding nitrones as the exclusive products, except for very hindered N-tert-alkyl substituted substrates. Using the ionic liquid [bmim]BF4 as a solvent resulted in a complete switch in reactivity and selectivity. N-methyl substituted imines gave the corresponding amides, while imines with bulkier substituents at nitrogen did not show any reactivity. An exception was the C-phenyl,N-tert-butyl imine—the only substrate that was oxidized to the corresponding oxaziridine, albeit with low conversion. The results reported herein reaffirm the oxidation of imines with UHP/MTO in MeOH as the method of choice for their interconversion to nitrones.
Full article
(This article belongs to the Section Catalysis in Organic and Polymer Chemistry)
►▼
Show Figures

Graphical abstract
Open AccessFeature PaperArticle
ZIF-67-Derived Co−N−C Supported Ni Nanoparticles as Efficient Recyclable Catalyst for Hydrogenation of 4-Nitrophenol
by
Juti Rani Deka, Diganta Saikia, Jia-Cheng Lin, Wan-Yu Chen, Hsien-Ming Kao and Yung-Chin Yang
Catalysts 2025, 15(4), 343; https://doi.org/10.3390/catal15040343 - 1 Apr 2025
Abstract
In this study, a novel, highly efficient, environment friendly, and low-cost nanocatalyst, denoted as Ni(x)@Co−N−C, was successfully developed by encapsulating Ni nanoparticles into N-doped porous carbon derived from ZIF-67. A variety of techniques including powder X-ray diffraction (XRD), nitrogen adsorption/desorption, scanning electron microscopy
[...] Read more.
In this study, a novel, highly efficient, environment friendly, and low-cost nanocatalyst, denoted as Ni(x)@Co−N−C, was successfully developed by encapsulating Ni nanoparticles into N-doped porous carbon derived from ZIF-67. A variety of techniques including powder X-ray diffraction (XRD), nitrogen adsorption/desorption, scanning electron microscopy (SEM), transmission electron microscopy (TEM), and X-ray photoelectron spectrometer (XPS) were used to characterize the prepared materials. The TEM images reveal that the nanoparticles were distributed homogeneously in the carbon support. The N atoms in the carbon support serve as the sites for the nucleation and uniform growth of Ni nanoparticles. The catalyst was used for the degradation of environmentally harmful 4-nitrophenol (4-NP) to 4-aminophenol (4-AP). Among all the catalysts investigated, Ni(10)@Co-N-C exhibited the highest catalytic activity for the hydrogenation of 4-NP, with a specific reaction rate of 6.1 × 10−3 s−1, activity parameter of 31 s−1g−1, and turn over frequency (TOF) of 1.78 × 1019 molecules gmetal−1s−1. On the other hand, the specific reaction rate and TOF value were 1.7 × 10−3 s−1 and 6.96 × 1018 molecules gmetal−1s−1, respectively, for Co−N−C. This suggests that Ni(10)@Co−N−C is about three times more catalytically active than the Co−N−C catalyst. The superb activity of Ni(10)@Co−N−C in comparison to Co−N−C can be ascribed to the homogeneous dispersion of small-sized Ni nanoparticles, the interconnected three-dimensional porous arrangement of the support Co−N−C, the presence of N atoms in the carbon framework that stabilize metal nanoparticles, and the synergistic electronic effect between Ni and Co. The Ni(10)@Co−N−C catalyst maintained consistent catalytic activity over multiple cycles, which suggests that porous N-containing carbon support can effectively prevent aggregation and leaching of metal nanoparticles. The ICP-AES analysis of the recycled Ni(10)@Co−N−C revealed a slight reduction in metal content compared to the fresh sample, suggesting almost negligible leaching of metal nanoparticles.
Full article
(This article belongs to the Special Issue Nanocatalysts for Degradation of Environmental Pollutants and Hydrogen Generation)
►▼
Show Figures

Graphical abstract
Open AccessFeature PaperArticle
Enhanced Nitrate Production via Electrocatalytic Nitric Oxide Oxidation Reaction over MnO2 with Different Crystal Facets
by
Xiaoyu Qin, Dongcai Shen, Quan Li, Xin Liu, Mingrui Wu and Wentai Wang
Catalysts 2025, 15(4), 342; https://doi.org/10.3390/catal15040342 - 1 Apr 2025
Abstract
The synthesis of nitrate (NO3−) via electrocatalytic nitric oxide oxidation reaction (NOOR) is a green and efficient strategy for nitrogen fixation, which has great advantages over conventional nitrate synthesis. Notably, it also presents a promising solution for the remediation of
[...] Read more.
The synthesis of nitrate (NO3−) via electrocatalytic nitric oxide oxidation reaction (NOOR) is a green and efficient strategy for nitrogen fixation, which has great advantages over conventional nitrate synthesis. Notably, it also presents a promising solution for the remediation of NO pollutants. In this study, the structure–performance correlations of α-, β-, and δ-MnO2 catalysts were investigated. These three polymorphs of MnO2 exhibited disparate surface chemistries, NO adsorption capabilities, and NOOR catalytic activities. In a 1.0 M KOH electrolyte, α-MnO2, characterized by its large-sized (2 × 2) one-dimensional tunnel structure, demonstrated the most outstanding NOOR catalytic performance, which achieved a remarkable NO3− yield of 665.2 mg·h−1·mgcat−1 at a potential of 1.9 V, along with excellent stability and durability. Furthermore, a Zn-NO system was constructed, employing α-MnO2 as the anode and a Zn plate as the cathode. This innovative setup integrated an energy storage system with NO electrochemical capture, yielding a NO3− production rate of 265.5 mg·h−1·mgcat−1. The density functional theory calculations confirm the NO adsorption performance and catalytic activity of three different crystal forms of MnO2.
Full article
(This article belongs to the Section Electrocatalysis)
►▼
Show Figures
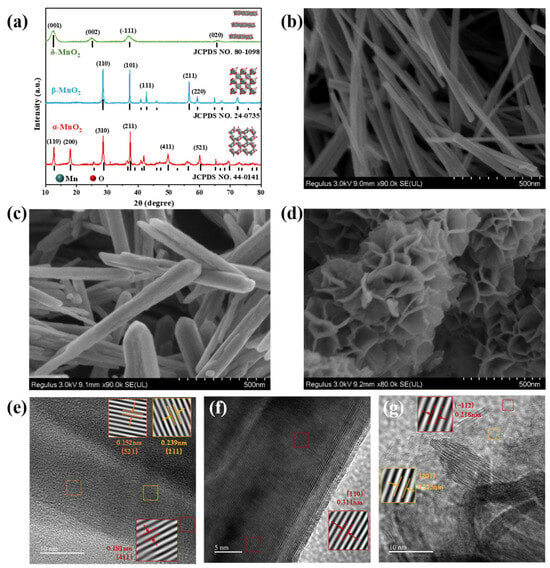
Figure 1
Open AccessArticle
Genome Mining of the Biocontrol Agent Trichoderma afroharzianum Unearths a Key Gene in the Biosynthesis of Anti-Fungal Volatile Sesquiterpenoids
by
Fang Zhang, Rui Ma, Yuyang Huang, Yang Cui, Qiong Zhou and Jingang Gu
Catalysts 2025, 15(4), 341; https://doi.org/10.3390/catal15040341 - 1 Apr 2025
Abstract
The volatile organic compounds (VOCs) in Trichoderma afroharzianum ACCC 33109 have the biological activities of both hydrolytic enzymes and antimicrobial peptides to mitigate attack by phytopathogens and spread over long distances in soil. However, the biosynthesis pathway of anti-fungal VOCs has not been
[...] Read more.
The volatile organic compounds (VOCs) in Trichoderma afroharzianum ACCC 33109 have the biological activities of both hydrolytic enzymes and antimicrobial peptides to mitigate attack by phytopathogens and spread over long distances in soil. However, the biosynthesis pathway of anti-fungal VOCs has not been elucidated yet. In this study, we identified 15 genes (TaTS1–15) coding for putative terpene synthase with low identities (<79.54%) to functionally characterized homologs through genome mining. Upon Fusarium induction, the relative expression levels of nine TaTS genes were up-regulated by up to 2793-fold (TaTS9). To verify the contribution of TaTS9 to the synthesis of anti-fungal VOCs, the TaTS9 knockout mutant strain was constructed and characterized by its antagonistic activities, transcript profiles, and VOC metabolomes. Heterologous expression of TaTS9 in Escherichia coli produced the target gene product, which converted the precursor farnesyl pyrophosphate (FPP) into β-cubenene (>90%) and γ-amorphene. Thus, TaTS9 was confirmed as the first β-cubenene synthase of Trichoderma, which catalyzes the biosynthesis of various sesquiterpenes with anti-fungal activities. This study provides insight into the key terpene synthase gene in the biosynthesis of anti-fungal sesquiterpenoids for potential applications in the agriculture and food industries.
Full article
(This article belongs to the Section Biocatalysis)
►▼
Show Figures
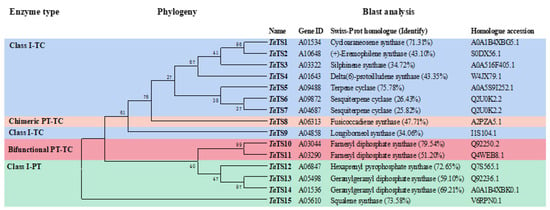
Figure 1

Journal Menu
► ▼ Journal Menu-
- Catalysts Home
- Aims & Scope
- Editorial Board
- Reviewer Board
- Topical Advisory Panel
- Instructions for Authors
- Special Issues
- Topics
- Sections & Collections
- Article Processing Charge
- Indexing & Archiving
- Editor’s Choice Articles
- Most Cited & Viewed
- Journal Statistics
- Journal History
- Journal Awards
- Society Collaborations
- Conferences
- Editorial Office
Journal Browser
► ▼ Journal BrowserHighly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Applied Nano, Catalysts, Materials, Nanomaterials, Polymers, Molecules
Application of Nanomaterials in Environmental Analysis
Topic Editors: Yonggang Zhao, Yun ZhangDeadline: 30 September 2025
Topic in
Energies, Materials, Catalysts, Metals, Hydrogen
Hydrogen—The New Energy Vector for the Transition of Industries "Hard to Abate"
Topic Editors: Pasquale Cavaliere, Geoffrey BrooksDeadline: 20 October 2025
Topic in
Applied Nano, Catalysts, Molecules, Nanomaterials, Water, Gels, Polymers
Water Purification and Catalytic Disintegration at the Nanoscale
Topic Editors: Michael Arkas, Ioannis Pashalidis, Dimitrios A. Giannakoudakis, Ioannis P. AnastopoulosDeadline: 30 November 2025
Topic in
Energies, Materials, Catalysts, Processes, Biomass
Advances in Biomass Conversion, 2nd Edition
Topic Editors: Jacek Grams, Agnieszka RuppertDeadline: 20 December 2025

Conferences
Special Issues
Special Issue in
Catalysts
Catalytic Effects in Electrochemical Energy Storage
Guest Editors: Bingsen Zhang, Ming Lu, Qinhua GuDeadline: 10 April 2025
Special Issue in
Catalysts
Nanomaterials in Environmental Catalysis
Guest Editors: Lixin Li, Lijie Xu, Langming Bai, Xin RenDeadline: 15 April 2025
Special Issue in
Catalysts
The State of the Art and Future Perspectives on Computational Catalysis
Guest Editors: Marta Castiñeira Reis, Carlo NerviDeadline: 15 April 2025
Special Issue in
Catalysts
Exclusive Papers in Green Photocatalysis from China
Guest Editors: Weilin Dai, Zhenfeng BianDeadline: 15 April 2025
Topical Collections
Topical Collection in
Catalysts
Catalytic Conversion and Utilization of Carbon-Based Energy
Collection Editor: Jian Qi
Topical Collection in
Catalysts
Catalytic Conversion of Biomass to Bioenergy
Collection Editors: Sergio Nogales Delgado, Juan Félix González, Simona M. Coman
Topical Collection in
Catalysts
Nanocatalysis Towards Energy Transition and Environmental Sustainability
Collection Editors: Michalis Konsolakis, Sónia Carabineiro
Topical Collection in
Catalysts
Catalysis in Advanced Oxidation Processes for Pollution Control
Collection Editors: Rongfu Huang, Fei Chang







