-
 Beeswax Alcohol (Bwa, Raydel) Protected Brain, Liver, and Kidney via Enhanced Antioxidant Activity Against Oxidative Damage in Lipid and Protein
Beeswax Alcohol (Bwa, Raydel) Protected Brain, Liver, and Kidney via Enhanced Antioxidant Activity Against Oxidative Damage in Lipid and Protein -
 Spinorphin Molecules as Opportunities for Incorporation into Spinorphin@AuNPs Conjugate Systems for Potential Sustained Targeted Delivery to the Brain
Spinorphin Molecules as Opportunities for Incorporation into Spinorphin@AuNPs Conjugate Systems for Potential Sustained Targeted Delivery to the Brain -
 Enhancing Tetrahydrocannabinol’s Therapeutic Efficacy in Inflammatory Bowel Disease: The Roles of Cannabidiol and the Cannabinoid 1 Receptor Allosteric Modulator ZCZ011
Enhancing Tetrahydrocannabinol’s Therapeutic Efficacy in Inflammatory Bowel Disease: The Roles of Cannabidiol and the Cannabinoid 1 Receptor Allosteric Modulator ZCZ011 -
 Uncovering the Mechanism of Action of Antiprotozoal Agents: A Survey on Photoaffinity Labeling Strategy
Uncovering the Mechanism of Action of Antiprotozoal Agents: A Survey on Photoaffinity Labeling Strategy
Journal Description
Pharmaceuticals
Pharmaceuticals
is a peer-reviewed, open access journal of medicinal chemistry and related drug sciences, published monthly online by MDPI. The Academy of Pharmaceutical Sciences (APS) is partners of Pharmaceuticals and their members receive a discount on the article processing charge.
- Open Access free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, SCIE (Web of Science), PubMed, PMC, Embase, CAPlus / SciFinder, and other databases.
- Journal Rank: JCR - Q1 (Pharmacology and Pharmacy) / CiteScore - Q2 (Pharmaceutical Science)
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 13.9 days after submission; acceptance to publication is undertaken in 3.4 days (median values for papers published in this journal in the second half of 2024).
- Recognition of Reviewers: reviewers who provide timely, thorough peer-review reports receive vouchers entitling them to a discount on the APC of their next publication in any MDPI journal, in appreciation of the work done.
- Testimonials: See what our editors and authors say about Pharmaceuticals.
- International Electronic Conference on Medicinal Chemistry (https://sciforum.net/series/ecmc/latest)
- Companion journals for Pharmaceuticals include: Pharmacoepidemiology, Psychoactives and Drugs and Drug Candidates.
Impact Factor:
4.3 (2023);
5-Year Impact Factor:
4.6 (2023)
Latest Articles
Investigation of c-Fos/c-Jun Signaling Pathways in Periostracum Cicadae’s Inhibition of EMT in Gastric Tissue
Pharmaceuticals 2025, 18(4), 537; https://doi.org/10.3390/ph18040537 (registering DOI) - 7 Apr 2025
Abstract
►
Show Figures
Background/Objectives: Periostracum Cicadae (PC) is commonly used to treat chronic atrophic gastritis (CAG), but its underlying mechanisms are unclear. We investigated the therapeutic effects, active ingredients and molecular mechanisms of PC on CAG. Methods: We analyzed the components in the serum
[...] Read more.
Background/Objectives: Periostracum Cicadae (PC) is commonly used to treat chronic atrophic gastritis (CAG), but its underlying mechanisms are unclear. We investigated the therapeutic effects, active ingredients and molecular mechanisms of PC on CAG. Methods: We analyzed the components in the serum extract of PC by UHPLC-Q-Orbitrap-MS/MS. Then, we used rat and cell models to assess the impact of PC on CAG and employed network pharmacology and bioinformatics to predict key targets and active ingredients. Finally, we confirmed hub targets through experiments and molecular docking. Results: A total of 22 components were identified in the PC extract-containing serum using UHPLC-Q-Orbitrap MS/MS. Network pharmacology combined with molecular docking revealed that the protective effect was primarily mediated by three compounds: (Z)-akuammidine, chicoric acid, and columbianadin. And we revealed that c-Fos/c-Jun signaling pathways were crucial in therapy. PC extract-containing serum inhibited the vitality, migration, invasion, and multiplication of MC cells (model cells for CAG), induced apoptosis, and caused G0/G1 phase cell cycle arrest. The expression level of tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), interleukin-1 beta (IL-1β) and gastrin 17 (G17) in the serum of CAG rats increased, while the expression level of pepsinogen I (PG I) and pepsinogen II (PG II) decreased. After 12 weeks of PC administration, these conditions were significantly improved. PC not only reduced the levels of antigen KI-67 (Ki67) and tumor protein p53 (P53) but also enhanced SRY-box Transcription Factor (SOX2). Simultaneously, PC down-regulated the expression of N-cadherin and Vimentin while up-regulating that of E-cadherin. Conclusions: PC inhibited epithelial–mesenchymal transition (EMT) via the c-Fos/c-Jun signaling pathway, thereby providing therapeutic benefits for CAG. Our study elucidates the mechanisms and material basis of PC in treating CAG, providing experimental evidence to support its clinical application.
Full article
Open AccessArticle
Hospitalized Cancer Patients with Opioid Management for Chemo-Induced Ulcerative Mucositis Lessens the Patients’ Overall Burden of Illness
by
Minu Ponnamma Mohan, Joel B. Epstein, Kapil S. Meleveedu, Parikshit Padhi, Roberto Pili and Poolakkad S. Satheeshkumar
Pharmaceuticals 2025, 18(4), 536; https://doi.org/10.3390/ph18040536 (registering DOI) - 6 Apr 2025
Abstract
Objectives: Mucositis is a debilitating side effect of cancer therapy that adversely affects quality of life, cost of care, and the outcome of cancer therapy. Oral mucositis-related pain may be treated with numerous modalities but often includes opioids. The effects of opiate
[...] Read more.
Objectives: Mucositis is a debilitating side effect of cancer therapy that adversely affects quality of life, cost of care, and the outcome of cancer therapy. Oral mucositis-related pain may be treated with numerous modalities but often includes opioids. The effects of opiate treatment on painful UM and its overall influence on the burden of illness (BOI) in cancer patients remain unknown. Methods: This study utilized the 2017 United States (US) National Inpatient Sample (NIS) database. The exposure was opioid treatment for chemo-induced ulcerative mucositis (UM), oral mucositis-induced pain, and the main outcomes included in-hospital mortality and BOI, length of hospital stays (LOS), and total hospital charges. Multivariable regression analysis was used to examine the relationship between outcomes and the key independent variable, opioid use, adjusting for propensity scores. Results: In the propensity score-adjusted analysis, UM patients with opioid treatment had 0.51 times lower total charges (95% CI: 0.42–0.76) and 0.67 times shorter LOS (95% CI: 0.51–0.87) than the UM patients without opioid treatment. However, there was no association between opioid treatment and in-hospital mortality. In the sensitivity analysis, the effect estimates were comparable in the propensity score-adjusted analysis, the decile-adjusted model, and the full model with the non-propensity score estimated method. Conclusions: Cancer patients with chemotherapy-induced UM-prescribed opioid analgesics for treating pain are associated with a lower BOI. Opioid pain medications are commonly provided to cancer survivors; estimating the BOI among them is crucial in supportive care research.
Full article
(This article belongs to the Special Issue Management of Pain in Oral Mucositis)
►▼
Show Figures

Graphical abstract
Open AccessReview
Artemisinin and Its Derivatives: Promising Therapeutic Agents for Age-Related Macular Degeneration
by
Chun Liu, Xiaoqin Liu and Junguo Duan
Pharmaceuticals 2025, 18(4), 535; https://doi.org/10.3390/ph18040535 (registering DOI) - 6 Apr 2025
Abstract
Age-related macular degeneration (AMD) is a leading cause of visual impairment and blindness in older adults. Its pathogenesis involves multiple factors, including aging, environmental influences, genetic predisposition, oxidative stress, metabolic dysfunction, and immune dysregulation. Currently, AMD treatment focuses primarily on wet AMD, managed
[...] Read more.
Age-related macular degeneration (AMD) is a leading cause of visual impairment and blindness in older adults. Its pathogenesis involves multiple factors, including aging, environmental influences, genetic predisposition, oxidative stress, metabolic dysfunction, and immune dysregulation. Currently, AMD treatment focuses primarily on wet AMD, managed through repeated intravitreal injections of anti-vascular endothelial growth factor (VEGF) therapies. While anti-VEGF agents represent a major breakthrough in wet AMD care, repeated injections may lead to incomplete responses or resistance in some patients, and carry a risk of progressive fibrosis. Artemisinin (ART) and its derivatives, originally developed as antimalarial drugs, exhibit a broad spectrum of pleiotropic activities beyond their established use, including anti-inflammatory, anti-angiogenic, antioxidant, anti-fibrotic, mitochondrial regulatory, lipid metabolic, and immunosuppressive effects. These properties position ART as a promising therapeutic candidate for AMD. A growing interest in ART-based therapies for AMD has emerged in recent years, with numerous studies demonstrating their potential benefits. However, no comprehensive review has systematically summarized the specific roles of ART and its derivatives in AMD pathogenesis and treatment. This paper aims to fill the knowledge gap by synthesizing the therapeutic efficacy and molecular mechanisms of ART and its derivatives in AMD, thereby providing a foundation for future investigations.
Full article
(This article belongs to the Section Natural Products)
►▼
Show Figures
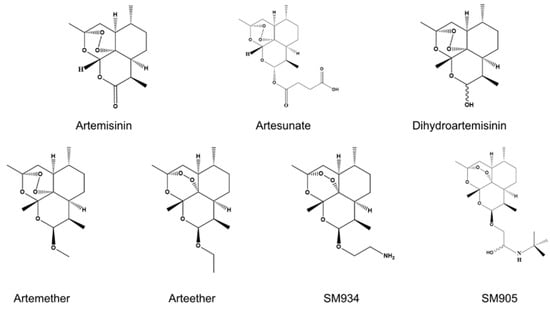
Figure 1
Open AccessArticle
In Vitro Evidence for the Efficacy of Manuka Honey and Its Components Against the Major Human Pathogenic Sporothrix Species
by
Andrea Reis Bernardes-Engemann, Fernando Almeida-Silva, Levi G. Cleare, Jefferson D. da Cruz, Jefferson Rocha de A. Silva, Walter Sotto M. Fernandes Neto, Rosely Maria Zancopé-Oliveira, Ana Claudia Fernandes Amaral, Joshua D. Nosanchuk and Rodrigo Almeida-Paes
Pharmaceuticals 2025, 18(4), 534; https://doi.org/10.3390/ph18040534 (registering DOI) - 6 Apr 2025
Abstract
Background/Objectives: While various clinical manifestations occur in sporotrichosis, cutaneous forms predominate. The recommended sporotrichosis treatment is itraconazole, an antifungal with certain restrictions. In recent years, the observation of reduced treatment effectiveness in some patients has arisen, possibly due to Sporothrix spp. resistance
[...] Read more.
Background/Objectives: While various clinical manifestations occur in sporotrichosis, cutaneous forms predominate. The recommended sporotrichosis treatment is itraconazole, an antifungal with certain restrictions. In recent years, the observation of reduced treatment effectiveness in some patients has arisen, possibly due to Sporothrix spp. resistance mechanisms. Consequently, there is a growing need for alternative therapeutic approaches. This study investigates the antifungal activity of manuka honey (MH) against pathogenic species of the genus Sporothrix. Methods: In this study, we assessed MH antifungal efficacy across concentrations ranging from 5% to 40% against 26 Sporothrix spp. isolates. In addition, its components were evaluated through chromatography and other in vitro techniques. Results: Minimum inhibitory concentrations of MH were found to be 15–40%, 10–15%, and 5–10% for Sporothrix brasiliensis, Sporothrix schenckii, and Sporothrix globosa, respectively. Purified methylglyoxal did not hinder Sporothrix growth. The MH antifungal potential was compromised through treatment with catalase or filtration through a 0.22 µm cellulose membrane. Chromatographic analysis of the volatile organic compounds (VOCs) present in MH identified 40 VOCs, including carbonyl compounds, alcohols, esters, aromatic hydrocarbons, heterocyclic compounds, terpenoids, and carboxylic acids. Additionally, two phenolic compounds were identified as potential markers for the authentication of MH, along with a disaccharide that may contribute to its antifungal activity. Conclusions: MH has demonstrated biological activity against the most significant Sporothrix species with pathogenic impact on humans. This suggests its consideration in future research endeavors focused on novel topical treatments for cutaneous sporotrichosis in both human and animal subjects.
Full article
(This article belongs to the Special Issue Fighting Tropical Neglected Diseases with Natural Products: New Approaches Against Ancient Diseases)
►▼
Show Figures
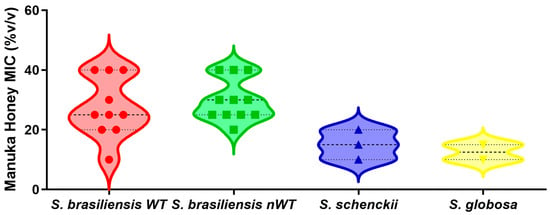
Figure 1
Open AccessArticle
The Value of Hydrogen Peroxide in Neurosurgery and Its Pathophysiological Effects in Human and Animal Brain Tissues
by
Violetta C. Spoeler, Markus Kipp, Daniel Dubinski, Joshua D. Bernstock, Artem Rafaelian, Svorad Trnovec, Cajetan I. Lang, Thomas M. Freiman, Sami Ridwan, Friedrich Prall, Florian Gessler and Sae-Yeon Won
Pharmaceuticals 2025, 18(4), 533; https://doi.org/10.3390/ph18040533 (registering DOI) - 6 Apr 2025
Abstract
Background: Hydrogen peroxide (H2O2) is a well-known hemostatic and antiseptic agent in neurosurgical practice. While there are concerns regarding the use of H2O2 due to its potential for neuronal damage, the pathophysiological effect on neuronal
[...] Read more.
Background: Hydrogen peroxide (H2O2) is a well-known hemostatic and antiseptic agent in neurosurgical practice. While there are concerns regarding the use of H2O2 due to its potential for neuronal damage, the pathophysiological effect on neuronal cells is not clearly understood. Methods: An online survey concerning the use of H2O2 was conducted in a board-certified platform, and an experimental study was designed to investigate the effect of H2O2 on neuronal and tumor cells. Brain tissues of mice and brain/tumor tissues of humans were irrigated with H2O2 3%, H2O2 1.5%, and NaCl 0.9%, and processed by bipolar coagulation. Tissue sections were obtained and stained with H&E and analyzed by the depth and degree of neuronal damage measured from the cortical surface (μm). Results: In total, 242 neurosurgeons participated in the survey, and 81% of neurosurgeons reported use of H2O2 in neurosurgical practice. however only 5% of the participants had a literature-based knowledge of the pathophysiological mechanism of H2O2. In total, eight mouse brain tissues, 21 human brain tissues, and seven human tumor tissues were processed and analyzed. The experimental study found that H2O2 caused vacuolization of neuronal tissue in mouse brain tissues, with a mean depth of damage of 343.7 ± 39.7 μm after 2 min and 460.1 ± 36.4 μm after 10 min exposure to H2O2 3% (p < 0.001). In human brain tissues, vacuolization was detected in sections exposed to H2O2 1.5% and 3%, with a mean depth of damage of 543.8 ± 304.5 μm and 859.0 ± 379 μm (p = 0.003). In the bipolar coagulation group, the mean depth of neuronal damage, of 2504 ± 1490 μm, was nearly three times greater than that in the H2O2 group (p < 0.001). Similar results were observed in human tumor tissues as well. Conclusions: H2O2 seems to cause less local damage on neuronal and tumor cells than conventional bipolar cauterization, suggesting it as a good alternative to be used for hemostasis and marginal tumor cell treatment. However, due to its potential risk for embolism, H2O2 should be used with caution.
Full article
(This article belongs to the Section Pharmacology)
►▼
Show Figures
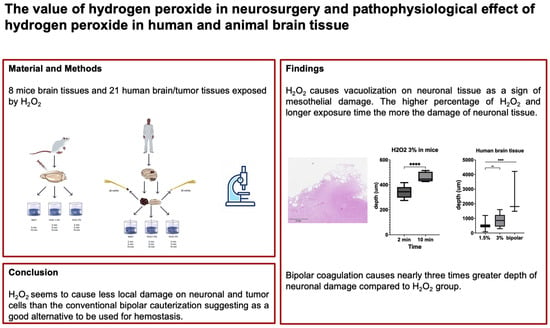
Graphical abstract
Open AccessReview
An Overview of the Effects of Lithium on Alzheimer’s Disease: A Historical Perspective
by
Marcia Radanovic, Monique Patricio Singulani, Vanessa de Jesus R. De Paula, Leda Leme Talib and Orestes Vicente Forlenza
Pharmaceuticals 2025, 18(4), 532; https://doi.org/10.3390/ph18040532 (registering DOI) - 5 Apr 2025
Abstract
Lithium was introduced into psychiatric practice in the late nineteenth century and has since become a standard treatment for severe psychiatric disorders, particularly those characterized by psychotic agitation. It remains the most effective agent for managing acute mania and preventing relapses in bipolar
[...] Read more.
Lithium was introduced into psychiatric practice in the late nineteenth century and has since become a standard treatment for severe psychiatric disorders, particularly those characterized by psychotic agitation. It remains the most effective agent for managing acute mania and preventing relapses in bipolar disorder. Despite potential adverse effects, lithium’s use should be carefully considered relative to other treatment options, as these alternatives may present distinct safety and tolerability profiles. The World Health Organization classifies lithium salts as ‘essential’ medications for inclusion in global healthcare systems. Over the past two decades, the growing recognition of lithium’s efficacy—extending beyond mood stabilization to include reducing suicide risk and inducing neuroprotection—has led to its incorporation into clinical practice guidelines. Current research, particularly from translational models, suggests that lithium’s pleiotropic effects benefit not only mental and brain health but also other organs and systems. This supports its potential as a therapeutic candidate for neurological conditions, particularly those associated with neurodegenerative processes. This article will discuss the historical background, discovery, and early experimentation of lithium in psychiatry. We will also review its mechanisms of action and discuss its potential in the treatment and prevention of neurodegenerative disorders, focusing on Alzheimer’s disease.
Full article
(This article belongs to the Special Issue Lithium in Psychiatric Therapy: Celebrating 75th Anniversary)
►▼
Show Figures
Graphical abstract
Open AccessArticle
Cytotoxic Effect of Escitalopram/Etoposide Combination on Etoposide-Resistant Lung Cancer
by
Serap Özkaya Gül, Beyzanur Şimşek, Fidan Yıldız and Esra Aydemir
Pharmaceuticals 2025, 18(4), 531; https://doi.org/10.3390/ph18040531 (registering DOI) - 5 Apr 2025
Abstract
Background: Antidepressants are a class of pharmaceuticals utilized for the management of many psychiatric disorders, including depression. A considerable number of antidepressants, particularly selective serotonin reuptake inhibitors (SSRIs), have been documented to demonstrate significant anticancer properties in various cancer cell lines. Objectives: The
[...] Read more.
Background: Antidepressants are a class of pharmaceuticals utilized for the management of many psychiatric disorders, including depression. A considerable number of antidepressants, particularly selective serotonin reuptake inhibitors (SSRIs), have been documented to demonstrate significant anticancer properties in various cancer cell lines. Objectives: The aim of this study was to evaluate the selective cytotoxic and apoptotic effects of escitalopram oxalate (ES) alone and in combination with etoposide (ET) on ET-resistant A549 (A549/90E) lung cancer cells. Methods: The cytotoxic effects of the drugs were determined by CCK-8, trypan blue, and neutral red assays. Apoptosis was observed by Annexin V fluorescein isothiocyanate (FITC)/PI and mitochondrial membrane potential (ΔΨm) assays. Moreover, the effects of the drugs, alone and in combination, on apoptosis-related proteins, caspase-3, PTEN, and resistance-related P-gP were determined by ELISA. The relationship between drugs and lung cancer was determined with protein–protein interaction (PPI) network analysis. Results: Our results revealed that ES significantly exerted cytotoxic effects on both wild-type and A549/90E cells compared with BEAS-2B cells. The IC50 values of 48.67 and 51.6 μg/mL obtained for ET and ES, respectively, at the end of 24 h of incubation for A549 cells were applied reciprocally for each cell by including BEAS-2B together with the 2xIC50 and ½ IC50 values. The results of each combination were statistically evaluated with combination indices (CIs) obtained using the Compusyn synergistic effect analysis program. Combination doses with a synergistic effect in A549 and A549/90E cells and an antagonistic effect in BEAS-2B cells have been determined as ½ IC50 for ET and ½ IC50 for ES. ET ½ IC50, ES ½ IC50, and an ET ½ IC50 + ES ½ IC50 combination caused 18.37%, 55.19%, and 57.55% death in A549 cells, whereas they caused 44.9%, 22.4%, and 51.94% death in A549/90E cells, respectively. In A549 cells, the combination of ES ½ IC50 and ET ½ IC50 caused increased levels of caspase-3 (p < 0.01) and P-gP (p < 0.001), while PTEN levels remained unchanged. The combination resulted in an increase in caspase-3 (p < 0.001) and PTEN (p < 0.001) amounts, alongside a decrease in P-gP (p < 0.01) levels in A549/90E cells. The death mechanism induced by the combination was found to be apoptotic by Annexin V-FITC and ΔΨm assays. Conclusions: Based on our findings, ES was observed to induce cytotoxic and apoptotic activities in A549/90E cells in vitro. ES in combination therapy is considered to be effective to overcome ET resistance by reducing the amount of P-gP in A549/90E cells.
Full article
(This article belongs to the Section Pharmacology)
►▼
Show Figures

Figure 1
Open AccessArticle
Mechanistic Advances in the Therapeutic Application of Bixin for Lung Inflammation In Vitro and In Vivo
by
Alexsandro Tavares Figueiredo-Junior, Bruno Clemente Brandão Marques, Douglas Galdino dos Santos, Wesley Leandro Gouveia, Raysa Magali Pillpe Meza, Luzineide Wanderley Tinoco, Lídia Moreira Lima, Samuel Santos Valenca and Manuella Lanzetti
Pharmaceuticals 2025, 18(4), 530; https://doi.org/10.3390/ph18040530 (registering DOI) - 5 Apr 2025
Abstract
Background: Nrf2 plays a key role in regulating the antioxidant response against oxidative stress. Therefore, it is imperative to examine the advantages of Nrf2 activation by new small molecules capable of inhibiting the Nrf2-Keap1 protein interaction that do not present electrophilic sites, since
[...] Read more.
Background: Nrf2 plays a key role in regulating the antioxidant response against oxidative stress. Therefore, it is imperative to examine the advantages of Nrf2 activation by new small molecules capable of inhibiting the Nrf2-Keap1 protein interaction that do not present electrophilic sites, since electrophilic compounds have intrinsic toxicity. The bixin pigment has been used as a form of treatment and prevention of several pathological conditions in animal models since it was described as an Nrf2 activator without electrophilic sites. This study aims to synthetize a soluble derivate KBx (potassium bixinate) and evaluate its ability to activate Nrf2/ARE in a model of exposure to cigarette smoke extract (CSE; in vitro) and intranasal LPS administration (in vivo). Methods: In the in vivo study, C57BL/6 mice were pretreated with 200 mg/kg of KBx (gavage) during 5 consecutive days and then challenged with 60 µg of LPS i.n. for 16 h. Bronchoalveolar lavage was collected to examine cytokines dosage. In the in vitro study, RAW 264.7 macrophages were exposed to CSE and post-treated with KBx to evaluate their ability to revert the redox imbalance caused by the stressor. Results: KBx was characterized using mass spectrometry (433.1778 m/z). KC levels were increased in the LPS group (p = 0.021), and KBx inhibited this (p = 0.001). IL-10 levels were decreased (p = 0.055) in the LPS group that was prevented when pretreated with KBx (p = 0.037). The in vitro study showed KBx to be a more potent derivate of bixin through its ability to intercept ROS formation with three-fold more potency, and it showed an anti-inflammatory propriety by reducing the nuclear translocation of p65 (p < 0.001). Conclusions: In conclusion, these data suggest that KBx was able to activate the Nrf2/ARE pathway and intercept ROS formation induced by CSE and LPS in both in vivo and in vitro studies.
Full article
(This article belongs to the Special Issue Bioactive Compounds From Plants and Their Therapeutic Applications in Lifestyle-Related Diseases)
►▼
Show Figures

Figure 1
Open AccessArticle
Uvarinol and Dichamanetin Derived from Uvaria chamae as Potential Dual-Site Inhibitors Against PBP2a in Methicillin Resistant Staphylococcus aureus: An In Silico Study
by
Emmanuel Ayodeji Agbebi, Shalom Oluwafunke Adeyemi, Adetola Ibukunoluwa Adewale, Omolara Seun Ajofoyinbo, Ezekiel Abiola Olugbogi, Oluwatoyin Mary Oyinloye, Iyadunni Adesola Anuoluwa, Timothy Oluwaseyi Agbebi, Basiru Olaitan Ajiboye and Babatunji Emmanuel Oyinloye
Pharmaceuticals 2025, 18(4), 529; https://doi.org/10.3390/ph18040529 (registering DOI) - 4 Apr 2025
Abstract
Background/Objectives: Methicillin-resistant Staphylococcus aureus (MRSA) is one of the resistant pathogenic microorganisms that poses a global health threat due to its resistance to β-lactam antibiotics where the protein penicillin-binding protein 2a (PBP2a) plays a crucial role in its resistance. This study explores
[...] Read more.
Background/Objectives: Methicillin-resistant Staphylococcus aureus (MRSA) is one of the resistant pathogenic microorganisms that poses a global health threat due to its resistance to β-lactam antibiotics where the protein penicillin-binding protein 2a (PBP2a) plays a crucial role in its resistance. This study explores the potential of phytochemicals from Uvaria chamae, a plant with known medicinal properties, to serve as dual-site inhibitors of PBP2a, targeting both the active and allosteric sites. Methods: Phytochemicals previously identified in U. chamae were subjected to molecular docking and molecular dynamics simulations to evaluate their binding affinities and stability at PBP2a’s active and allosteric sites. The compounds’ pharmacokinetic profiles were predicted in silico using SwissADME tools. Root-mean-square deviation (RMSD), radius of gyration, and binding free energy were analyzed for dynamic stability. Results: Among the evaluated compounds, Uvarinol and Dichamanetin demonstrated high binding affinities compared to the co-crystallized ligand and standard antibiotics like ceftaroline. Uvarinol exhibited the highest binding affinity at both sites, with a docking score of −14.94 kcal/mol and a predicted inhibition constant (Ki) of 0.01 nM. Molecular dynamics simulations further confirmed the robust stability of Uvarinol and Dichamanetin, as indicated by consistently lower RMSD values relative to the co-crystallized ligand. Pharmacokinetic predictions revealed favorable drug-likeness and low toxicity, although Uvarinol showed limited gastrointestinal absorption. Conclusions: Uvarinol and Dichamanetin show promise as dual-site PBP2a inhibitors, offering a novel strategy to combat MRSA resistance. Their structural and pharmacokinetic properties make them viable candidates for further development, though experimental validation and formulation optimization are necessary to overcome bioavailability challenges.
Full article
(This article belongs to the Section Biopharmaceuticals)
►▼
Show Figures
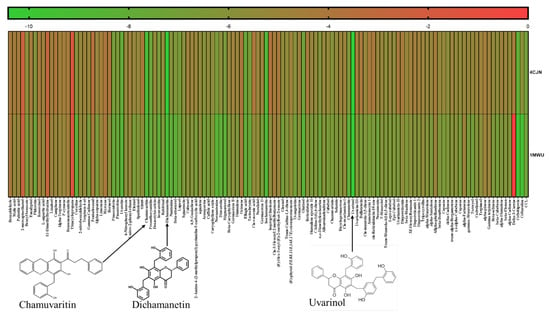
Figure 1
Open AccessArticle
Comparison Study of the Safety Profile of Olaparib Versus Niraparib: Analysis of Real-World Data from EudraVigilance
by
Desirèe Speranza, Fausto Omero, Vincenzo Cianci, Mariapia Marafioti, Carla Infurna, Patrizia Carroccio, Edoardo Spina, Maria Antonietta Barbieri, Emanuela Esposito, Nicola Silvestris and Mariacarmela Santarpia
Pharmaceuticals 2025, 18(4), 528; https://doi.org/10.3390/ph18040528 (registering DOI) - 4 Apr 2025
Abstract
Background: Olaparib and niraparib are poly (ADP-ribose) polymerase inhibitors (PARPi) used primarily for the treatment of ovarian cancer. While both drugs have demonstrated efficacy in clinical trials, their safety profiles, particularly in real-world clinical settings, remain to be fully elucidated. Objectives: This study
[...] Read more.
Background: Olaparib and niraparib are poly (ADP-ribose) polymerase inhibitors (PARPi) used primarily for the treatment of ovarian cancer. While both drugs have demonstrated efficacy in clinical trials, their safety profiles, particularly in real-world clinical settings, remain to be fully elucidated. Objectives: This study aimed to (i) characterize the adverse drug reactions (ADRs) associated with olaparib and niraparib as reported in the EudraVigilance database, (ii) compare the frequency of the ADRs occurring during treatment with the two drugs, and (iii) compare post-marketing safety data with those from clinical trials. Methods: A retrospective analysis was performed using data from the EudraVigilance database (2017–2024), focusing on individual case safety reports (ICSRs) related to olaparib and niraparib. Descriptive statistics and disproportionality analysis were performed to compare the frequency and severity of reported ADRs. Results: Both olaparib and niraparib had common ADRs including nausea, vomiting, anemia, thrombocytopenia, and fatigue. However, olaparib was associated with a higher risk of myelodysplastic syndrome (MDS), acute myeloid leukemia (AML), and interstitial lung disease, while niraparib had a higher incidence of gastrointestinal events and thrombocytopenia. Our analysis demonstrates that some specific ADRs, including peripheral neuropathy with niraparib, were reported at higher frequencies compared to clinical trials. The incidence of serious ADRs, including hospitalizations and life-threatening events, was higher with niraparib than with olaparib. Conclusions: This study highlights significant differences in the safety profiles of olaparib and niraparib, with implications for clinical decision-making. Continuous monitoring and personalized management of ADRs are essential to optimize patient outcomes.
Full article
(This article belongs to the Section Pharmacology)
►▼
Show Figures
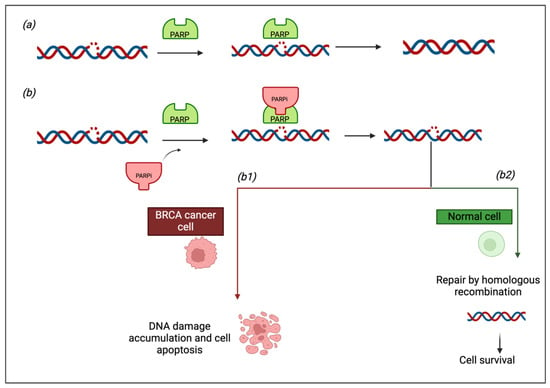
Figure 1
Open AccessArticle
Central Insulin-like Growth Factor-1 Treatment Enhances Working and Reference Memory by Reducing Neuroinflammation and Amyloid Beta Deposition in a Rat Model of Sporadic Alzheimer’s Disease
by
Joanna Dunacka, Beata Grembecka, Irena Majkutewicz and Danuta Wrona
Pharmaceuticals 2025, 18(4), 527; https://doi.org/10.3390/ph18040527 - 4 Apr 2025
Abstract
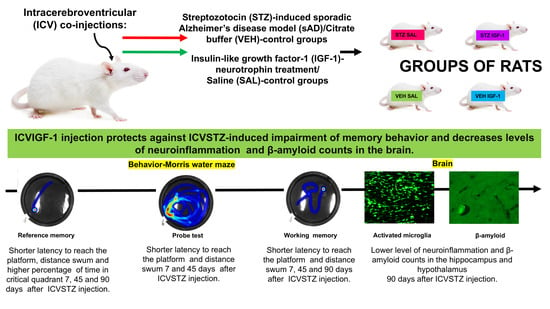
Background/Objectives: Brain insulin resistance is a potential causal factor for dementia in Alzheimer’s disease (AD). Insulin-like growth factor-1 (IGF-1), a neurotrophin, plays a key role in central insulin signaling and neuroprotection. Intracerebrovenitricular (ICV) administration of streptozotocin (STZ) disrupts insulin signal transduction, leading
[...] Read more.
Background/Objectives: Brain insulin resistance is a potential causal factor for dementia in Alzheimer’s disease (AD). Insulin-like growth factor-1 (IGF-1), a neurotrophin, plays a key role in central insulin signaling and neuroprotection. Intracerebrovenitricular (ICV) administration of streptozotocin (STZ) disrupts insulin signal transduction, leading to brain insulin resistance, which may mimic the early pathophysiological changes in sporadic AD (sAD). In this study, we investigated whether restoring insulin signaling through ICV injection of IGF-1 could ameliorate spatial memory deficits during sAD progression in a rat model induced by ICV STZ injection. Methods: Male Wistar rats (n = 40) were subjected to double ICV injections of STZ (0.75 mg/kg/ventricle, days 2 and 4) and IGF-1 (1 μg/single injection, days 1 and 3), and placed at the Morris water maze (MWM) at baseline, 7, 45 and 90 days after injections. Reference (days 1–3 and day 4 MWM)) and working (days 5–8 MWM) memory, microglia activation (CD68+ cells), and amyloid β (Aβ) deposition (immunohistochemistry) were measured. Results: We found that ICVIGF-1 administration protected working memory demonstrated as (1) reduced latency to reach the platform, and reduced swimming distance in trials 3 (p < 0.05) and 4 (p < 0.01) on days 45 and 90 post-injection and (2) a short-term (up to 45 days post-injection) enhancement of reference memory, manifested by a reduction in swimming distance and latency (p < 0.05). Furthermore, IGF-1 treatment reduced neuroinflammation in CA2 (p < 0.05) and Aβ deposition in CA1(p < 0.01) of the hippocampus. Conclusions: Central IGF-1 attenuates spatial memory deficits in the ICVSTZ-induced sAD model by reducing neuroinflammation and Aβ accumulation in the hippocampus.
Full article
(This article belongs to the Special Issue New Insights into Therapy for Alzheimer’s and Other Neurodegenerative Diseases)
►▼
Show Figures
Graphical abstract
Open AccessArticle
Efficacy of RADA16-Based Self-Assembling Peptides on Wound Healing: A Meta-Analysis of Preclinical Animal Studies
by
Jiaju Lu, Liuting Chen, Zeyue Sun and Zhimou Yang
Pharmaceuticals 2025, 18(4), 526; https://doi.org/10.3390/ph18040526 - 3 Apr 2025
Abstract
Objectives: This analysis aims to provide evidence supporting the feasibility of clinical application of self-assembling peptides for skin wound healing. Methods: This review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. PubMed, Web of Science,
[...] Read more.
Objectives: This analysis aims to provide evidence supporting the feasibility of clinical application of self-assembling peptides for skin wound healing. Methods: This review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. PubMed, Web of Science, and Cochrane Library were searched (up to June 17, 2024). The primary outcome, wound closure rate at 7 and 14 days post-injury, was pooled using a random-effects meta-analysis. The risk of bias (ROB) assessment and meta-analysis were performed using the Systematic Review Centre for Laboratory animal Experimentation (SYRCLE)’s ROB tool for animal studies and RevMan software. Results: A total of 502 unique records were identified from our search, with 12 experimental animal studies meeting the prespecified inclusion criteria (n = 272 animals). The RADA16 interventions promoted wound closure rate compared to controls (saline or no treatment group) in both diabetic and non-diabetic animal models (Mean Difference (MD) = 11.25, 95% Confidence Interval (CI): 5.73 to 16.78, p < 0.0001; MD = 9.48, 95% CI: 4.75 to 14.22, p < 0.0001 at 7 and 14 days post-injury, respectively). Healing was further enhanced using RADA16-based functional self-assembling peptides compared to RADA16 group in both diabetic and non-diabetic animal models (MD = 27.25, 95% CI: 22.68 to 31.83, p < 0.00001; MD = 29.11, 95% CI: 24.30 to 33.91, p < 0.00001 at 7 and 14 days after injury, respectively). The ROB was uncertain for most studies due to insufficient reporting. Conclusions: RADA16-based self-assembling peptides, particularly those modified with functional peptide motifs, represent a promising treatment for non-diabetic and diabetic wounds in pre-clinical studies, and translation to the clinical domain appears warranted.
Full article
(This article belongs to the Special Issue Peptide Biomaterials for Pharmaceutical Applications)
►▼
Show Figures

Graphical abstract
Open AccessReview
Can Focused Ultrasound Overcome the Failure of Chemotherapy in Treating Pediatric Diffuse Intrinsic Pontine Glioma Due to a Blood–Brain Barrier Obstacle?
by
Silvana Filieri, Morena Miciaccia, Domenico Armenise, Olga Maria Baldelli, Anselma Liturri, Savina Ferorelli, Anna Maria Sardanelli, Maria Grazia Perrone and Antonio Scilimati
Pharmaceuticals 2025, 18(4), 525; https://doi.org/10.3390/ph18040525 (registering DOI) - 3 Apr 2025
Abstract
Background: The blood–brain barrier (BBB) plays an important role in regulating homeostasis of the central nervous system (CNS), and it is an obstacle for molecules with a molecular weight higher than 500 Da seeking to reach it, making many drugs ineffective simply
[...] Read more.
Background: The blood–brain barrier (BBB) plays an important role in regulating homeostasis of the central nervous system (CNS), and it is an obstacle for molecules with a molecular weight higher than 500 Da seeking to reach it, making many drugs ineffective simply because they cannot be delivered to where they are needed. As a result, crossing the BBB remains the rate-limiting factor in brain drug delivery during the treatment of brain diseases, specifically tumors such as diffuse intrinsic pontine glioma (DIPG), a highly aggressive pediatric tumor with onset in the pons Varolii, the middle portion of the three contiguous parts of the brainstem, located above the medulla and below the midbrain. Methods: Currently, radiotherapy (RT) relieves DIPG symptoms but chemotherapy drugs do not lead to significant results as they do not easily cross the BBB. Focused ultrasound (FUS) and microbubbles (MBs) can temporarily open the BBB, facilitating radiotherapy and the entry of drugs into the CNS. A patient-derived xenograft DIPG model exposed to high-intensity focalized ultrasound (HIFU) or low-intensity focalized ultrasound (LIFU) combined with MBs was treated with doxorubicin, panobinostat, olaparib, ONC201 (Dordaviprone®) and anti-PD1. Panobinostat has also been used in children with diffuse midline glioma, a broad class of brain tumors to which DIPG belongs. Results: Preliminary studies were performed using FUS to temporarily open the BBB and allow a milder use of radiotherapy and facilitate the passage of drugs through the BBB. The data collected show that after opening the BBB with FUS and MBs, drug delivery to the CNS significantly improved. Conclusions: FUS associated with MBs appears safe and feasible and represents a new strategy to increase the uptake of drugs in the CNS and therefore enhance their effectiveness. This review reports pre-clinical and clinical studies performed to demonstrate the usefulness of FUS in patients with DIPG treated with some chemotherapy. The papers reviewed were published in PubMed until the end of 2024 and were found using a combination of the following keywords: diffuse intrinsic pontine glioma (DIPG), DIPG H3K27-altered, blood–brain barrier and BBB, focused ultrasound (FUS) and radiotherapy (RT).
Full article
(This article belongs to the Section Radiopharmaceutical Sciences)
►▼
Show Figures
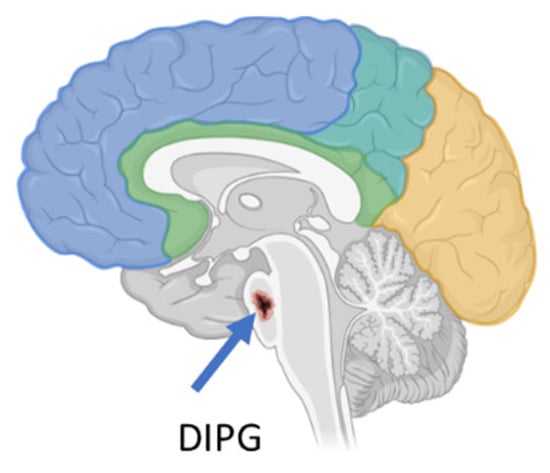
Figure 1
Open AccessArticle
Elucidating the Synergistic Effect of the PrimeC Combination for Amyotrophic Lateral Sclerosis in Human Induced Pluripotent Stem Cell-Derived Motor Neurons and Mouse Models
by
Shiran Salomon-Zimri, Nitai Kerem, Gabriel R. Linares, Niva Russek-Blum, Justin K. Ichida and Ferenc Tracik
Pharmaceuticals 2025, 18(4), 524; https://doi.org/10.3390/ph18040524 - 3 Apr 2025
Abstract
Background: Amyotrophic lateral sclerosis (ALS) is a multifactorial neurodegenerative disease characterized by the involvement of multiple pathways and mechanisms. The complexity of its pathophysiology is reflected in the diverse hypotheses relating to its underlying causes. Given this intricate interplay of processes, a combination
[...] Read more.
Background: Amyotrophic lateral sclerosis (ALS) is a multifactorial neurodegenerative disease characterized by the involvement of multiple pathways and mechanisms. The complexity of its pathophysiology is reflected in the diverse hypotheses relating to its underlying causes. Given this intricate interplay of processes, a combination therapy approach offers a promising strategy. Combination therapies have demonstrated significant success in treating complex diseases, where they aim to achieve synergistic therapeutic effects and reduce drug dosage. PrimeC is an oral combination treatment composed of a patented novel formulation consisting of specific and unique doses of two well-characterized drugs (ciprofloxacin and celecoxib). It aims to synergistically inhibit the progression of ALS by addressing key elements of its pathophysiology. Objectives: Demonstrating the synergistic effect of the PrimeC combination compared to each of its individual components, celecoxib and ciprofloxacin, and assessing its ability to improve the drug concentration profile and efficacy. Methods: The efficacy of the PrimeC combination was assessed in a survival assay using human induced pluripotent stem cell (iPSC)-derived motor neurons. Additionally, a drug profiling study was conducted, measuring drug levels in the brain and serum of C57BL mice treated with a single compound versus the combination. Results: Motor neurons modeling ALS treated with the PrimeC combination exhibited better survival rates compared to treatment with either individual compound alone. The enhanced efficacy of the combination was further supported by a drug concentration profiling study in rodents, demonstrating that the PrimeC combination resulted in increased ciprofloxacin concentrations in both brain tissue and serum—highlighting the optimized interaction and synergistic potential of its two comprising agents. Conclusions: Our findings support the potential of combination therapy as an effective strategy for ALS treatment. Specifically, the PrimeC combination demonstrated promising therapeutic effects, providing a strong rationale for its ongoing development as a targeted treatment for ALS.
Full article
(This article belongs to the Special Issue New Insights into Therapy for Alzheimer’s and Other Neurodegenerative Diseases)
►▼
Show Figures

Figure 1
Open AccessReview
Rho Kinase (ROCK) Inhibitors in the Treatment of Glaucoma and Glaucoma Surgery: A Systematic Review of Early to Late Phase Clinical Trials
by
Jit Kai Tan, Peng Tee Khaw and Christin Henein
Pharmaceuticals 2025, 18(4), 523; https://doi.org/10.3390/ph18040523 - 3 Apr 2025
Abstract
Background/Objectives: Primary open-angle glaucoma (POAG) is an anterior optic neuropathy that can lead to irreversible vision loss if untreated. Prostaglandin analogues are the first-line treatment, but new drug classes, such as rho kinase (ROCK) inhibitors, are being explored. This review evaluates the
[...] Read more.
Background/Objectives: Primary open-angle glaucoma (POAG) is an anterior optic neuropathy that can lead to irreversible vision loss if untreated. Prostaglandin analogues are the first-line treatment, but new drug classes, such as rho kinase (ROCK) inhibitors, are being explored. This review evaluates the efficacy and safety of ROCK inhibitors in treating POAG based on completed trials, comparing results with available natural history data and identifying areas for further research. Methods: A systematic database search was conducted in Ovid MEDLINE and Ovid Embase on 5 April 2022 using the following keywords: ‘glaucoma’, ‘rho kinase inhibitor’, ‘rho-kinase inhibitor’, ‘rock inhibitor’, ‘ripasudil’, ‘netarsudil’, and ‘fasudil’. Abstracts were screened for relevant studies and results summarized in tables. Results: The analysis of trials conducted for ROCK inhibitors reveals that they are a safe and efficacious drug to treat POAG, demonstrating non-inferiority to existing medical treatments. Comparison of data to natural history studies was inconclusive due to the lack of natural history studies and their limitations. The results showed ROCK inhibitors to be effective when combined with existing medical treatments. However, questions remain regarding the optimal dosage, patient selection, and cost-effectiveness. Outcome measures for future trials should be expanded to include additional methods of monitoring disease progression as well as patient quality-of-life. Conclusions: ROCK inhibitors have emerged with a favorable safety profile, efficaciously attenuating intraocular pressure. To elucidate their long-term therapeutic value and safety comprehensively, further independent, large-scale, prospective randomized controlled trials are warranted. Such studies are pivotal to augment our understanding of this emergent medication class.
Full article
(This article belongs to the Special Issue 20th Anniversary of Pharmaceuticals—Advances in Pathophysiology, Pharmacology and Neuroprotection in Glaucoma)
Open AccessReview
Fibroblast Activation Protein Inhibitor (FAPI)-Based Theranostics
by
William Serumula, Venesen Pillay, Bawinile Hadebe and Mariza Vorster
Pharmaceuticals 2025, 18(4), 522; https://doi.org/10.3390/ph18040522 (registering DOI) - 3 Apr 2025
Abstract
Fibroblast activation protein (FAP) is a serine protease selectively expressed in cancer-associated fibroblasts (CAFs), fibrotic tissues, and areas of active tissue remodeling, making it an attractive target for diagnostic imaging across a spectrum of disease. FAP inhibitors (FAPIs) labeled with PET tracers have
[...] Read more.
Fibroblast activation protein (FAP) is a serine protease selectively expressed in cancer-associated fibroblasts (CAFs), fibrotic tissues, and areas of active tissue remodeling, making it an attractive target for diagnostic imaging across a spectrum of disease. FAP inhibitors (FAPIs) labeled with PET tracers have rapidly advanced as a novel imaging modality with broad clinical applications that offers several advantages, including rapid tumor accumulation, low background uptake, and high tumor-to-background ratios. In oncology, FAPI PET has demonstrated excellent performance in visualizing a wide range of malignancies, including those with low glycolytic activity, such as pancreatic cancer, cholangiocarcinoma, and certain sarcomas. Its high sensitivity and specificity for the stromal component enables improved tumor delineation, staging, and response assessment. Additionally, the potential to guide theranostic approaches, where the same tracer can be labeled with therapeutic radionuclides, positions FAPI as a key player in precision oncology. Beyond oncology, FAPI PET has shown promise in imaging conditions characterized by fibrotic and inflammatory processes. In the cardiovascular field, FAPI PET imaging is being investigated for its ability to detect myocardial fibrosis and active cardiac remodeling, crucial in conditions like heart failure, post-myocardial infarction remodeling, and hypertrophic cardiomyopathy. This review highlights the expanding clinical applications of FAPI-based PET imaging across oncology, inflammation, and cardiovascular disease. While the current data are promising, further large-scale studies and multicenter trials are essential to validate these findings and establish standardized protocols. The versatility and broad applicability of FAPI PET underscore its potential as a transformative tool in precision medicine.
Full article
(This article belongs to the Special Issue The Medical Applications of Novel PET Radiopharmaceuticals)
►▼
Show Figures

Graphical abstract
Open AccessArticle
New Cannabinoids and Chlorin-Type Metabolites from the Flowers of Cannabis sativa L.: A Study on Their Neuroblastoma Activity
by
Tuan-Quoc Nguyen, Hyo-Shin Park, Sun-Hyeong Choi, Da-Yun Hong, Jae-Yong Cheon, Young-Mi Lee, Chul-Min Kim, Jong-Ki Hong, Seo-Jeong Oh, Man-Soo Cho, Jang-Hoon Kim, Eun-Sol Lee, Jungwon Seo and Hyun-Ju Jung
Pharmaceuticals 2025, 18(4), 521; https://doi.org/10.3390/ph18040521 - 3 Apr 2025
Abstract
Background/Objectives: Cannabis sativa has been utilized for medical purposes for thousands of years. It continues to be recognized as a plant with an extensive variety of medicinal and nutraceutical uses today. In this study, a chemical investigation of the flowers of C.
[...] Read more.
Background/Objectives: Cannabis sativa has been utilized for medical purposes for thousands of years. It continues to be recognized as a plant with an extensive variety of medicinal and nutraceutical uses today. In this study, a chemical investigation of the flowers of C. sativa isolated by using a variety of chromatographic techniques led to the isolation of eleven compounds. These purified compounds were evaluated for antitumor activity against SK-N-SH neuroblastoma cells. Methods: The compounds were isolated by using chromatographic techniques. Their structures were identified by the examination of spectroscopic methods, including 1D (1H, 13C, and DEPT) and 2D (COSY, HSQC, HMBC, and NOESY) nuclear magnetic resonance (NMR) spectra and mass spectrum, together with the comparison to those reported previously in the literature. The evaluation of toxicity on SK-N-SH cells was performed by the MTT method. Results: Eleven compounds were isolated from the flowers of C. sativa, including two new compounds, namely cannabielsoxa (1), 132-hydroxypheophorbide c ethyl ester (2), and six known cannabinoids (6–11), together with the first isolation of chlorin-type compounds: pyropheophorbide A (3), 132-hydroxypheophorbide b ethyl ester (4), and ligulariaphytin A (5) from this plant. The results also demonstrated that cannabinoid compounds had stronger inhibitory effects on neuroblastoma cells than chlorin-type compounds. Conclusions: The evaluation of the biological activities of compounds showed that compounds 4–10 could be considered as the potential compounds for antitumor effects against neuroblastomas. This is also highlighted by using docking analysis. Additionally, the results of this study also suggest that these compounds have the potential to be developed into antineuroblastoma products.
Full article
(This article belongs to the Special Issue Pharmacologically Active Compounds from Plants)
►▼
Show Figures
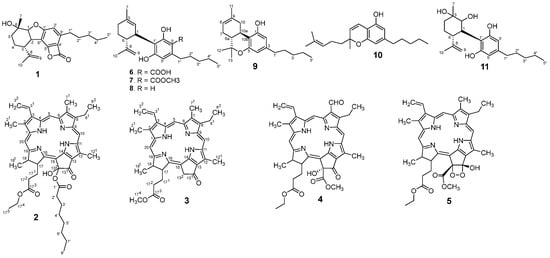
Figure 1
Open AccessReview
Advancements in Osteosarcoma Therapy: Overcoming Chemotherapy Resistance and Exploring Novel Pharmacological Strategies
by
Mahmoud Zhra, Shahid Akhtar Akhund and Khalid S. Mohammad
Pharmaceuticals 2025, 18(4), 520; https://doi.org/10.3390/ph18040520 - 3 Apr 2025
Abstract
Osteosarcoma is recognized as the most prevalent primary bone malignancy, primarily affecting children and adolescents. It is characterized by its aggressive behavior and high metastatic potential, which often leads to poor patient outcomes. Despite advancements in surgical techniques and chemotherapy regimens, the prognosis
[...] Read more.
Osteosarcoma is recognized as the most prevalent primary bone malignancy, primarily affecting children and adolescents. It is characterized by its aggressive behavior and high metastatic potential, which often leads to poor patient outcomes. Despite advancements in surgical techniques and chemotherapy regimens, the prognosis for patients with osteosarcoma remains unsatisfactory, with survival rates plateauing over the past few decades. A significant barrier to effective treatment is the development of chemotherapy resistance, which complicates the management of the disease and contributes to high rates of recurrence. This review article aims to provide a comprehensive overview of recent advancements in osteosarcoma therapy, particularly in overcoming chemotherapy resistance. We begin by discussing the current standard treatment modalities, including surgical resection and conventional chemotherapy agents such as methotrexate, doxorubicin, and cisplatin. While these approaches have been foundational in managing osteosarcoma, they are often limited by adverse effects and variability in efficacy among patients. To address these challenges, we explore novel pharmacological strategies that aim to enhance treatment outcomes. This includes targeted therapies focusing on specific molecular alterations in osteosarcoma cells and immunotherapeutic approaches designed to harness the body’s immune system against tumors. Additionally, we review innovative drug delivery systems that aim to improve the bioavailability and efficacy of existing treatments while minimizing toxicity. The review also assesses the mechanisms underlying chemotherapy resistance, such as drug efflux mechanisms, altered metabolism, and enhanced DNA repair pathways. By synthesizing current research findings, we aim to highlight the potential of new therapeutic agents and strategies for overcoming these resistance mechanisms. Ultimately, this article seeks to inform future research directions and clinical practices, underscoring the need for continued innovation in treating osteosarcoma to improve patient outcomes and survival rates.
Full article
(This article belongs to the Special Issue Osteosarcomas: Treatment Strategies, 2nd Edition)
►▼
Show Figures

Graphical abstract
Open AccessReview
Mechanisms of Action of AGuIX as a Pan-Cancer Nano-Radiosensitizer: A Comprehensive Review
by
Clémentine Aubrun, Tristan Doussineau, Léna Carmès, Aurélien Meyzaud, Fabien Boux, Sandrine Dufort, Adeline Delfour, Olivier De Beaumont, Céline Mirjolet and Géraldine Le Duc
Pharmaceuticals 2025, 18(4), 519; https://doi.org/10.3390/ph18040519 - 2 Apr 2025
Abstract
Objective: This review provides an overview of the current knowledge regarding the mechanisms of action of AGuIX, a clinical-stage theranostic nano-radiosensitizer composed of gadolinium. It covers the steps following the administration, from the internalization in tumor cells to the interaction with X-rays and
[...] Read more.
Objective: This review provides an overview of the current knowledge regarding the mechanisms of action of AGuIX, a clinical-stage theranostic nano-radiosensitizer composed of gadolinium. It covers the steps following the administration, from the internalization in tumor cells to the interaction with X-rays and the subsequent physical, chemical, biological, and immunological events. Results: After intravenous injection, AGuIX accumulates in tumors through the enhanced permeability and retention (EPR) effect, and its specific retention properties allow its persistence in tumors for several days. At the cellular level, the nanomedicine is internalized by endocytic processes and mainly located in the cytoplasm, especially in lysosomes. AGuIX enhances the effects of radiotherapy (RT) at several levels, starting from radiation–matter interactions to a chemical stage of reactive oxygen species (ROS) production, followed by a cascade of biological events leading to tumor cell death and immune response. Indeed, AGuIX induces a local increase in radiation dose deposition through the emission of Auger electrons, leading to a subsequent increase in ROS generation. AGuIX also impacts RT-induced biological mechanisms, including DNA damage and cell death mechanisms such as apoptosis, autophagic cell death, and ferroptosis. Last, the combination of AGuIX and RT stimulates an antitumor immune response through the induction of immunogenic cell death (ICD), the activation of dendritic and T cells, and the reprogramming of tumor-associated macrophages (TAMs) into a pro-inflammatory phenotype. Conclusions: AGuIX is a clinical-stage nanoparticle (NP) intravenously administered with pan-cancer potential due to its specific biodistribution properties and a strong ability to amplify RT-induced mechanisms.
Full article
(This article belongs to the Section Radiopharmaceutical Sciences)
►▼
Show Figures

Graphical abstract
Open AccessSystematic Review
Pharmacological Efficacy of Intravenous Magnesium in Attenuating Remifentanil-Induced Postoperative Hyperalgesia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
by
En-Bo Wu, Kuen-Lin Wu, Wei-Ti Hsu, Wei-Chin Yuan and Kuen-Bao Chen
Pharmaceuticals 2025, 18(4), 518; https://doi.org/10.3390/ph18040518 - 1 Apr 2025
Abstract
Background/Objectives: Remifentanil-based anesthesia is linked to opioid-induced hyperalgesia (OIH), increasing postoperative pain and analgesic requirements. Magnesium, an N-methyl-D-aspartate (NMDA) receptor antagonist, might alleviate OIH. We aimed to assess whether intravenous magnesium reduces postoperative pain, analgesic requirements, and hyperalgesia in adults receiving remifentanil-based
[...] Read more.
Background/Objectives: Remifentanil-based anesthesia is linked to opioid-induced hyperalgesia (OIH), increasing postoperative pain and analgesic requirements. Magnesium, an N-methyl-D-aspartate (NMDA) receptor antagonist, might alleviate OIH. We aimed to assess whether intravenous magnesium reduces postoperative pain, analgesic requirements, and hyperalgesia in adults receiving remifentanil-based anesthesia. Methods: We searched PubMed, Embase, the Cochrane Library, and Web of Science (1 December 2024) for randomized controlled trials (RCTs) comparing intravenous magnesium vs. placebo. Risk of bias was evaluated with the Cochrane RoB 2 tool, and random-effects meta-analyses were conducted. GRADE was used to assess evidence quality. Primary outcomes were postoperative analgesic requirements and pain scores; secondary outcomes included intraoperative remifentanil consumption, shivering, postoperative nausea/vomiting (PONV), extubation time, hypotension, and bradycardia. PROSPERO registration: CRD42024609911. Results: Twenty-two RCTs (n = 1362) met eligibility. Magnesium significantly decreased 24 h analgesic requirements (standardized mean difference [SMD] −1.51; 95% confidence interval [CI] −2.15 to −0.87; p < 0.0001) and pain scores (SMD −0.61; 95% CI −0.90 to −0.32; p < 0.0001), with benefits persisting up to 48 h. It also reduced intraoperative remifentanil use (SMD −0.52), shivering (odds ratio [OR] 0.25), and PONV (OR 0.66), without prolonging extubation or increasing hypotension/bradycardia risk. High heterogeneity, potential publication bias, and moderate-to-very-low evidence certainty warrant caution. Conclusions: Intravenous magnesium appears beneficial in remifentanil-based anesthesia, but further large-scale, methodologically robust trials are needed to confirm optimal and clarify safety profiles across diverse surgical populations.
Full article
(This article belongs to the Section Pharmacology)
►▼
Show Figures

Figure 1

Journal Menu
► ▼ Journal Menu-
- Pharmaceuticals Home
- Aims & Scope
- Editorial Board
- Reviewer Board
- Topical Advisory Panel
- Instructions for Authors
- Special Issues
- Topics
- Sections & Collections
- Article Processing Charge
- Indexing & Archiving
- Editor’s Choice Articles
- Most Cited & Viewed
- Journal Statistics
- Journal History
- Journal Awards
- Society Collaborations
- Editorial Office
Journal Browser
► ▼ Journal BrowserHighly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
IJMS, JCM, Molecules, Pharmaceuticals, Pharmaceutics, Chemistry
Opioids and Their Receptors: Present and Emerging Concepts in Opioid Drug Discovery
Topic Editors: Mariana Spetea, Hideaki Fujii, Jay P. McLaughlinDeadline: 30 April 2025
Topic in
Biomedicines, JNT, Pharmaceutics, Polymers, Nanomaterials, Pharmaceuticals, Biophysica
Applications of Polymers and Polymer Nanomaterials in Drug Delivery and Nanomedicine
Topic Editors: Stanislav Rangelov, Emi HaladjovaDeadline: 31 May 2025
Topic in
Antibiotics, Biomedicines, JCM, Pharmaceuticals, Pharmaceutics
Challenges and Future Prospects of Antibacterial Therapy
Topic Editors: Kwang-sun Kim, Zehra EdisDeadline: 30 June 2025
Topic in
Applied Microbiology, Microorganisms, Pharmaceuticals, Pharmaceutics, Foods
Probiotics: New Avenues
Topic Editors: Daniela Machado, José Carlos AndradeDeadline: 15 August 2025

Conferences
Special Issues
Special Issue in
Pharmaceuticals
Blood–Brain-Barrier-Penetrating Therapeutics Targeting Intrinsically Disordered Proteins in Neurodegenerative Diseases
Guest Editor: Chih Hung LoDeadline: 10 April 2025
Special Issue in
Pharmaceuticals
Natural-Based Skincare Solutions
Guest Editor: Célia CabralDeadline: 15 April 2025
Special Issue in
Pharmaceuticals
New Insights of Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors in Biomedical Applications
Guest Editors: Afif Nakhleh, Bshara Mansour, Naim ShehadehDeadline: 15 April 2025
Special Issue in
Pharmaceuticals
Pharmacotherapy for Neuropathic Pain
Guest Editors: Vittoria Borgonetti, Nicoletta GaleottiDeadline: 20 April 2025
Topical Collections
Topical Collection in
Pharmaceuticals
The Story of Successful Drugs and Recent FDA-Approved Molecules
Collection Editors: Maria Emília De Sousa, Jean Jacques Vanden Eynde, Klaus Kopka, Annie Mayence, Joachim Jose, Guangshun Wang
Topical Collection in
Pharmaceuticals
Therapeutic Agents for Neurological Disorders
Collection Editor: Damian Holsinger
Topical Collection in
Pharmaceuticals
Feature Review Collection of Natural Products
Collection Editors: Daniela De Vita, Thomas Efferth, Martina Bortolami
Topical Collection in
Pharmaceuticals
Feature Review Collection in Medicinal Chemistry
Collection Editors: Alessandra Ammazzalorso, Luís M. T. FrijaConference Reports
Pharmaceuticals 2023, 16(3), 432; https://doi.org/10.3390/ph16030432
Pharmaceuticals 2022, 15(4), 388; https://doi.org/10.3390/ph15040388








