-
 Evolution of Alzheimer’s Disease Therapeutics: From Conventional Drugs to Medicinal Plants, Immunotherapy, Microbiotherapy and Nanotherapy
Evolution of Alzheimer’s Disease Therapeutics: From Conventional Drugs to Medicinal Plants, Immunotherapy, Microbiotherapy and Nanotherapy -
 Alginate Hydrogel Beads with a Leakproof Gold Shell for Ultrasound-Triggered Release
Alginate Hydrogel Beads with a Leakproof Gold Shell for Ultrasound-Triggered Release -
 Exploring the Potential of Gold Nanoparticles in Proton Therapy: Mechanisms, Advances, and Clinical Horizons
Exploring the Potential of Gold Nanoparticles in Proton Therapy: Mechanisms, Advances, and Clinical Horizons
Journal Description
Pharmaceutics
Pharmaceutics
is a peer-reviewed, open access journal on the science and technology of pharmaceutics and biopharmaceutics, and is published monthly online by MDPI. The Spanish Society of Pharmaceutics and Pharmaceutical Technology (SEFIG), Pharmaceutical Solid State Research Cluster (PSSRC), Academy of Pharmaceutical Sciences (APS) and Korean Society of Pharmaceutical Sciences and Technology (KSPST) are affiliated with Pharmaceutics and their members receive a discount on the article processing charges.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, SCIE (Web of Science), PubMed, PMC, Embase, CAPlus / SciFinder, and other databases.
- Journal Rank: JCR - Q1 (Pharmacology and Pharmacy) / CiteScore - Q1 (Pharmaceutical Science)
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 15.5 days after submission; acceptance to publication is undertaken in 2.9 days (median values for papers published in this journal in the second half of 2024).
- Recognition of Reviewers: reviewers who provide timely, thorough peer-review reports receive vouchers entitling them to a discount on the APC of their next publication in any MDPI journal, in appreciation of the work done.
- Companion journals for Pharmaceutics include: Future Pharmacology, Journal of Pharmaceutical and BioTech Industry and Medicines.
Impact Factor:
4.9 (2023);
5-Year Impact Factor:
5.5 (2023)
Latest Articles
Tumor Treatment by Nano-Photodynamic Agents Embedded in Immune Cell Membrane-Derived Vesicles
Pharmaceutics 2025, 17(4), 481; https://doi.org/10.3390/pharmaceutics17040481 (registering DOI) - 7 Apr 2025
Abstract
Non-invasive phototherapy includes modalities such as photodynamic therapy (PDT) and photothermal therapy (PTT). When combined with tumor immunotherapy, these therapeutic approaches have demonstrated significant efficacy in treating advanced malignancies, thus attracting considerable attention from the scientific community. However, the progress of these therapies
[...] Read more.
Non-invasive phototherapy includes modalities such as photodynamic therapy (PDT) and photothermal therapy (PTT). When combined with tumor immunotherapy, these therapeutic approaches have demonstrated significant efficacy in treating advanced malignancies, thus attracting considerable attention from the scientific community. However, the progress of these therapies is hindered by inherent limitations and potential adverse effects. Recent findings indicate that certain therapeutic strategies, including phototherapy, can induce immunogenic cell death (ICD), thereby opening new avenues for the integration of phototherapy with tumor immunotherapy. Currently, the development of biofilm nanomaterial-encapsulated drug delivery systems has reached a mature stage. Immune cell membrane-encapsulated nano-photosensitizers hold great promise, as they can enhance the tumor immune microenvironment. Based on bioengineering technology, immune cell membranes can be designed according to the tumor immune microenvironment, thereby enhancing the targeting and immune properties of nano-photosensitizers. Additionally, the space provided by the immune cell membrane allows for the co-encapsulation of immunotherapeutic agents and chemotherapy drugs, achieving a synergistic therapeutic effect. At the same time, the timing of photodynamic therapy (PDT) can be precisely controlled to regulate the action timing of both immunotherapeutic and chemotherapy drugs. This article summarizes and analyzes current research based on the aforementioned advancements.
Full article
(This article belongs to the Special Issue Smart Nanomedicine for Cancer Diagnosis and Therapy)
►
Show Figures
Open AccessArticle
Preparation and Therapeutic Evaluation of Engineered Semaglutide and Statin–Lipid Conjugate-Based Nanoparticle
by
Kyeong-Ju Lee, Seong-Bin Yang, Jae-Hyeon Lee, Bison Seo, Hyung-Sik Won and Jooho Park
Pharmaceutics 2025, 17(4), 480; https://doi.org/10.3390/pharmaceutics17040480 (registering DOI) - 7 Apr 2025
Abstract
Background: Fatty liver disease and obesity are among the most prevalent health conditions in modern society and have recently garnered significant attention. Semaglutide, a well-known anti-obesity drug, has been widely used for diabetes and obesity treatment; however, nanotherapeutics utilizing semaglutide have not
[...] Read more.
Background: Fatty liver disease and obesity are among the most prevalent health conditions in modern society and have recently garnered significant attention. Semaglutide, a well-known anti-obesity drug, has been widely used for diabetes and obesity treatment; however, nanotherapeutics utilizing semaglutide have not yet been developed. Methods: A novel statin–lipid conjugate was synthesized using rosuvastatin and ursodeoxycholic acid, a liver-protective agent. This conjugate was then formulated with semaglutide through hydrophobic interactions to create a new nanoparticle system. The physicochemical properties of the nanoparticles were analyzed, and their therapeutic efficacy was evaluated in a high-fat diet (HFD)-induced animal model. Results: The statin–lipid conjugate was successfully synthesized, forming novel nanoparticles with semaglutide in an aqueous solution. These nanoparticles exhibited distinct properties compared to conventional semaglutide formulations. In animal experiments, the treatment group demonstrated a 30.24% reduction in body weight and a 46.80% improvement in liver function markers compared to the control group. Conclusions: This study introduces a novel semaglutide-based nanoparticle (SRLC NP) system that overcomes key limitations of conventional semaglutide therapy by providing enhanced bioavailability, extended circulation time, and improved cellular uptake. These findings highlight the potential of SRLC NPs as a clinically translatable nanotherapeutic approach for more effective, sustained, and patient-friendly obesity and fatty liver disease treatment.
Full article
(This article belongs to the Section Nanomedicine and Nanotechnology)
►▼
Show Figures

Graphical abstract
Open AccessReview
Applications of Matrix Metalloproteinase-9-Related Nanomedicines in Tumors and Vascular Diseases
by
Xuying Li and Zhuping Xu
Pharmaceutics 2025, 17(4), 479; https://doi.org/10.3390/pharmaceutics17040479 (registering DOI) - 7 Apr 2025
Abstract
Matrix metalloproteinase-9 (MMP-9) is implicated in tumor progression and vascular diseases, contributing to angiogenesis, metastasis, and extracellular matrix degradation. This review comprehensively examines the relationship between MMP-9 and these pathologies, exploring the underlying molecular mechanisms and signaling pathways involved. Specifically, we discuss the
[...] Read more.
Matrix metalloproteinase-9 (MMP-9) is implicated in tumor progression and vascular diseases, contributing to angiogenesis, metastasis, and extracellular matrix degradation. This review comprehensively examines the relationship between MMP-9 and these pathologies, exploring the underlying molecular mechanisms and signaling pathways involved. Specifically, we discuss the contribution of MMP-9 to tumor epithelial–mesenchymal transition, angiogenesis, and metastasis, as well as its involvement in a spectrum of vascular diseases, including macrovascular, cerebrovascular, and ocular vascular diseases. This review focuses on recent advances in MMP-9-targeted nanomedicine strategies, highlighting the design and application of responsive nanoparticles for enhanced drug delivery. These nanotherapeutic strategies leverage MMP-9 overexpression to achieve targeted drug release, improved tumor penetration, and reduced systemic toxicity. We explore various nanoparticle platforms, such as liposomes and polymer nanoparticles, and discuss their mechanisms of action, including degradation, drug release, and targeting specificity. Finally, we address the challenges posed by the heterogeneity of MMP-9 expression and their implications for personalized therapies. Ultimately, this review underscores the diagnostic and therapeutic potential of MMP-9-targeted nanomedicines against tumors and vascular diseases.
Full article
(This article belongs to the Section Nanomedicine and Nanotechnology)
►▼
Show Figures

Graphical abstract
Open AccessArticle
Nanostructured Lipid Carriers (NLC)-Based Topical Formulation of Hesperidin for Effective Treatment of Psoriasis
by
Anita Rani, Rajwinder Kaur, Afaf Aldahish, Rajalakshimi Vasudevan, Prasanalakshmi Balaji, Chander Parkash Dora, Balakumar Chandrasekaran, Thakur Gurjeet Singh and Rahul Sharma
Pharmaceutics 2025, 17(4), 478; https://doi.org/10.3390/pharmaceutics17040478 (registering DOI) - 7 Apr 2025
Abstract
Background: Various routes of drug administration are available for psoriasis treatment. However, there is an urgent need for novel and improved therapeutic options. Hence, our study aimed to develop a nanostructured lipid carrier (NLC) gel of hesperidin (HPD) using a systemic QbD approach
[...] Read more.
Background: Various routes of drug administration are available for psoriasis treatment. However, there is an urgent need for novel and improved therapeutic options. Hence, our study aimed to develop a nanostructured lipid carrier (NLC) gel of hesperidin (HPD) using a systemic QbD approach for an effective treatment of psoriasis. Methods: Initially, HPD-NLC was optimized with independent variables (drug content, amount of liquid lipid, total lipid, and surfactant concentration) using Box–Behnken Design to assess dependent variables (particle size, size distribution, and entrapment efficiency). HPD-NLC was developed using the high-shear homogenization technique. The characteristics of nanoformulation such as particle size, morphology [transmission electron microscopy (TEM) and differential scanning calorimetry (DSC)], crystallinity [powder X-ray diffraction (XRD)], and chemical interactions [Fourier transform infrared spectroscopy (FTIR)], the drug entrapment efficiency (%EE), and the drug release were investigated. Franz-diffusion cell was utilized to perform in vitro diffusion study, and an imiquimod-induced psoriasis model was used for in vivo study. Results: The optimized HPD-NLC exhibited a spherical shape with particle size of 125.7 nm, polydispersity index (PDI) of 0.36, and entrapment efficiency of 52.26% w/w. Further, different techniques validated the reduced crystallinity of the hesperidin. The in vitro diffusion study highlighted the sustained and anomalous diffusion of the drug from NLC gel. In the in vivo study, the HPD-NLC-Gel-treated group displayed normal skin with minimal keratosis, while the drug-loaded gel group exhibited signs of hyperkeratosis and parakeratosis signs. Conclusions: HPD-NLC gel showed promising advancement in nanotechnology-based psoriasis treatment and the results of this study open the door for the application of topical HPD-NLC-Gel clinically.
Full article
(This article belongs to the Special Issue Nanocomposite Hydrogels and Their Application in Drug Delivery and Tissue Engineering)
►▼
Show Figures

Figure 1
Open AccessArticle
Carbon Dots as a Fluorescent Nanosystem for Crossing the Blood–Brain Barrier with Plausible Application in Neurological Diseases
by
Catarina Araújo, Raquel O. Rodrigues, Manuel Bañobre-López, Adrián M. T. Silva and Rui S. Ribeiro
Pharmaceutics 2025, 17(4), 477; https://doi.org/10.3390/pharmaceutics17040477 (registering DOI) - 6 Apr 2025
Abstract
Background/Objectives: The development of effective therapies for brain disorders is highly correlated with the ability of drugs or nanosystems to cross the blood–brain barrier (BBB), which has been limited. Recently, carbon dots (CDs) have been receiving attention to be used as BBB-crossing
[...] Read more.
Background/Objectives: The development of effective therapies for brain disorders is highly correlated with the ability of drugs or nanosystems to cross the blood–brain barrier (BBB), which has been limited. Recently, carbon dots (CDs) have been receiving attention to be used as BBB-crossing theranostic agents due to their inherent advantages, such as low size, excellent biocompatibility, high quantum yield (QY), tunable fluorescence, high drug loading, and relatively easy synthesis at low cost. The aim of this study was to design CDs with precisely controlled fluorescence properties for advanced bioimaging and an in-depth assessment of BBB permeability. Methods: CDs were synthesized using a microwave-assisted approach, optimized through microwaves’ irradiation time, and employing citric acid, urea, and sodium fluoride as precursors. The optimized sample was labeled as NF-CD. Results: A comprehensive physicochemical, photoluminescence, and biological characterization revealed the ability of NF-CD to diffuse across a neuromimetic-BBB model, mainly due to their small size (average diameter of 4.0 ± 1.1 nm), exhibiting excitation-dependent fluorescence in the blue and green wavelengths, high biocompatibility and QY, and exceptional photostability. Conclusions: Owing to the exceptional fluorescence characteristics and biological compatibility, NF-CD presents promising opportunities in theranostic applications, particularly in brain-targeted bioimaging, nanocarrier-based drug and immunotherapy delivery, early-stage diagnostics, and personalized medicine. NF-CD’s ability to cross the BBB further underscores the relevance of pioneering nanomaterial-based strategies for neurological disorder diagnostics and precision-targeted therapeutic interventions. Overall, this research contributes to the broader field of nanotechnology-driven biomedical advancements, fostering innovations in neurological diagnostics and therapeutic delivery systems.
Full article
(This article belongs to the Section Nanomedicine and Nanotechnology)
►▼
Show Figures

Figure 1
Open AccessSystematic Review
Role of Gut Microbiota and Metabolomics in Predicting Response to Vedolizumab in Inflammatory Bowel Disease: A Systematic Review
by
Vaidota Malinauskiene, Elena Cijauskaite, Goda Sadauskaite and Ieva Stundiene
Pharmaceutics 2025, 17(4), 476; https://doi.org/10.3390/pharmaceutics17040476 (registering DOI) - 6 Apr 2025
Abstract
Background: This review explores the impact of gut microbiota profiles in predicting the response to anti-integrin biologic therapy, particularly vedolizumab, in inflammatory bowel disease (IBD) patients. IBD, encompassing Crohn’s disease and ulcerative colitis, is a chronic inflammatory condition with a growing prevalence
[...] Read more.
Background: This review explores the impact of gut microbiota profiles in predicting the response to anti-integrin biologic therapy, particularly vedolizumab, in inflammatory bowel disease (IBD) patients. IBD, encompassing Crohn’s disease and ulcerative colitis, is a chronic inflammatory condition with a growing prevalence linked to industrialization and lifestyle changes. Disruption in the gut microbiota balance, characterized by reduced diversity and altered short-chain fatty acid (SCFA) production, is associated with IBD and its symptoms. Current pharmacological treatments target healing and remission, with vedolizumab offering a gut-selective treatment approach. Methods: A search of the literature was performed on the relationship between anti-integrin treatment and the microbiome profile in IBD. Articles were examined from the PubMed, Medline, Cochrane, and Web of Science databases. Results: This review identified five human studies investigating the relationship between gut microbiome composition, SCFAs, and response to vedolizumab, revealing an increased abundance of beneficial bacteria and levels of SCFAs like butyrate in remission cases. Despite promising findings, the small sample sizes and limited scope of the existing studies highlight the need for larger, comprehensive research. Conclusions: This review underscores the potential of gut microbiome and metabolite profiling as non-invasive biomarkers for IBD severity and treatment outcomes, advocating for personalized therapeutic strategies to enhance efficacy. The insights gained could lead to novel diagnostic and treatment modalities, although further validation is necessary to fully understand the intricate connections between gut microbiota and IBD prognosis.
Full article
(This article belongs to the Section Biologics and Biosimilars)
►▼
Show Figures
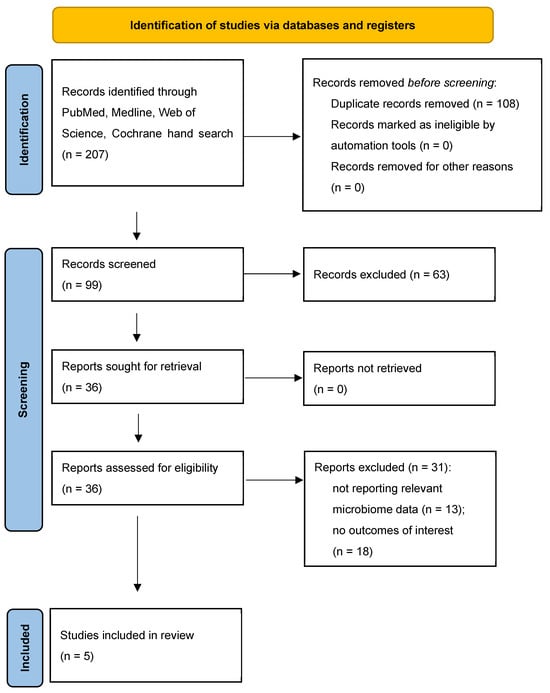
Figure 1
Open AccessArticle
Evaluation of Olive Oil-Based Formulations Loaded with Baricitinib for Topical Treatment of Alopecia Areata
by
Negar Beirampour, Mireia Mallandrich, Paola Bustos-Salgado, Valeri Domínguez-Villegas, Núria Garrós, Roya Mohammadi-Meyabadi, Beatriz Clares-Naveros, Maria Nuria Romero-Olid, Francisco J. Pérez-Cano, Marina Girbal, Maria José Rodríguez-Lagunas, Joaquim Suñer-Carbó and Ana Cristina Calpena
Pharmaceutics 2025, 17(4), 475; https://doi.org/10.3390/pharmaceutics17040475 (registering DOI) - 5 Apr 2025
Abstract
Background: Alopecia areata is an autoimmune disorder that causes hair loss in clumps about the size and shape of a quarter. The estimated prevalence of the disorder is approximately 1 in 1000 people, with a lifetime risk of approximately 2 percent. One of
[...] Read more.
Background: Alopecia areata is an autoimmune disorder that causes hair loss in clumps about the size and shape of a quarter. The estimated prevalence of the disorder is approximately 1 in 1000 people, with a lifetime risk of approximately 2 percent. One of the systemic therapies for alopecia areata consists of the use of glucocorticoids or immunosuppressants. Methods: Baricitinib (BCT) is a Janus kinase (JAK) 1 and 2 selective inhibitor used as an immunosuppressant drug. In this study, three olive oil BCT formulations (Oil A, Oil B, and Oil C, which differ in their content in squalene, tocopherol, tyrosol, and hydroxytyrosol) have been developed for topical delivery. The formulations were physicochemically characterized and the in vitro drug release and ex vivo permeation through human skin tissues were assessed. Results: The results showed nearly identical viscosity across all three formulations, exhibiting Newtonian behavior. The mathematical modeling used to describe the drug release profiles was the one-site binding hyperbola for all formulations. Oil-based formulations showed a slow BCT penetration into human skin. Skin integrity remained intact during the experiments, with no signs of irritation or alterations observed. In addition, all the formulations proved their efficacy in vivo. Conclusions: Among the formulations, Oil A demonstrated the highest ability retention capacity (Qr = 1875 ± 124.32 ng/cm2) in the skin, making it an excellent candidate for further investigation in the treatment of alopecia areata.
Full article
(This article belongs to the Section Drug Delivery and Controlled Release)
►▼
Show Figures

Figure 1
Open AccessArticle
Formulation and In Vitro Evaluation of Matrix Tablets Containing Ketoprofen–Beta Cyclodextrin Complex for Enhanced Rheumatoid Arthritis Therapy: Experimental and Computational Insights
by
Monica Stamate Cretan, Lacramioara Ochiuz, Vlad Ghizdovat, Monica Molcalut, Maricel Agop, Carmen Anatolia Gafițanu, Alexandra Barsan (Bujor), Mousa Sha’at and Ciprian Stamate
Pharmaceutics 2025, 17(4), 474; https://doi.org/10.3390/pharmaceutics17040474 (registering DOI) - 5 Apr 2025
Abstract
Background: Rheumatoid arthritis is a chronic autoimmune disease that leads to severe disability and requires improved therapeutic strategies to optimize anti-inflammatory treatment. This study aimed to address this challenge by developing and characterizing an extended-release polymer matrix tablet containing ketoprofen and a ketoprofen–β-cyclodextrin
[...] Read more.
Background: Rheumatoid arthritis is a chronic autoimmune disease that leads to severe disability and requires improved therapeutic strategies to optimize anti-inflammatory treatment. This study aimed to address this challenge by developing and characterizing an extended-release polymer matrix tablet containing ketoprofen and a ketoprofen–β-cyclodextrin complex with enhanced therapeutic properties. The objective was to improve inflammation management and therapeutic outcomes using a novel delivery system based on the inclusion of the active substance in cyclodextrin complexes. Methods: Tablets were formulated using ketoprofen and ketoprofen–β-cyclodextrin complexes combined with hydrophilic polymers such as Carbopol® 971P NF, Kollidon® VA 64, and MethocelTM K4M. The complexes were obtained via the coprecipitation method to improve bioavailability. The kinetics of the release of ketoprofen, ketoprofen–β-cyclodextrin complex (2:1), and ketoprofen–β-cyclodextrin complex (1:1) from the tablets were investigated in vitro in artificial gastric and intestinal fluids, and drug release profiles were established. Advanced mathematical models were used to describe the nonlinear behavior of the drug–polymer systems. Results: The inclusion of ketoprofen in the β-cyclodextrin complexes was confirmed, revealing distinct release profiles. Tablets (K-3 F-3) containing the 1:1 complex showed rapid release (96.2% in 4–7 h), while tablets (K-1 F-4) containing free ketoprofen released 76% over 9–11 h. Higher polymer concentrations slowed the release due to gel barrier formation. Pharmacotechnical and stability tests supported their suitability as extended-release forms. A multifractal modeling approach described the release dynamics, treating the polymer–drug matrix as a complex system, with release curves characterized by variations in the fractal dimension and resolution. Conclusions: Specific hydrophilic polymer combinations effectively prolonged ketoprofen release. The developed matrix tablets, which were evaluated via in vitro studies and mathematical modeling, show promise for improving therapeutic outcomes and patient compliance during rheumatoid arthritis treatment.
Full article
(This article belongs to the Section Drug Delivery and Controlled Release)
►▼
Show Figures
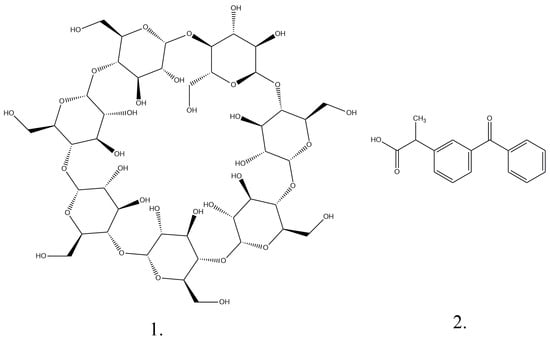
Figure 1
Open AccessArticle
Monitoring of Nutrients, Metabolites, IgG Titer, and Cell Densities in 10 L Bioreactors Using Raman Spectroscopy and PLS Regression Models
by
Morandise Rubini, Julien Boyer, Jordane Poulain, Anaïs Berger, Thomas Saillard, Julien Louet, Martin Soucé, Sylvie Roussel, Sylvain Arnould, Murielle Vergès, Fabien Chauchard-Rios and Igor Chourpa
Pharmaceutics 2025, 17(4), 473; https://doi.org/10.3390/pharmaceutics17040473 (registering DOI) - 4 Apr 2025
Abstract
Background: Chinese hamster ovary (CHO) cell metabolism is complex, influenced by nutrients like glucose and glutamine and metabolites such as lactate. Real-time monitoring is necessary for optimizing culture conditions and ensuring consistent product quality. Raman spectroscopy has emerged as a robust process analytical
[...] Read more.
Background: Chinese hamster ovary (CHO) cell metabolism is complex, influenced by nutrients like glucose and glutamine and metabolites such as lactate. Real-time monitoring is necessary for optimizing culture conditions and ensuring consistent product quality. Raman spectroscopy has emerged as a robust process analytical technology (PAT) tool due to its non-invasive, in situ capabilities. This study evaluates Raman spectroscopy for monitoring key metabolic parameters and IgG titer in CHO cell cultures. Methods: Raman spectroscopy was applied to five 10 L-scale CHO cell cultures. Partial least squares (PLS) regression models were developed from four batches, including one with induced cell death, to enhance robustness. The models were validated against blind test sets. Results: PLS models exhibited high predictive accuracy (R2 > 0.9). Glucose and IgG titer predictions were reliable (RMSEP = 0.51 g/L and 0.12 g/L, respectively), while glutamine and lactate had higher RMSEP due to lower concentrations. Specific Raman bands contributed to the specificity of glucose, lactate, and IgG models. Predictions for viable (VCD) and total cell density (TCD) were less accurate due to the absence of direct Raman signals. Conclusions: This study confirms Raman spectroscopy’s potential for real-time, in situ bioprocess monitoring without manual sampling. Chemometric analysis enhances model robustness, supporting automated control systems. Raman data could enable continuous feedback regulation of critical nutrients like glucose, ensuring consistent critical quality attributes (CQAs) in biopharmaceutical production.
Full article
(This article belongs to the Section Pharmaceutical Technology, Manufacturing and Devices)
►▼
Show Figures

Figure 1
Open AccessArticle
Topical Administration of Vitamin D2 Combined with Colloidal Silver Nanoparticles Promotes Wound Repair and Protection Against Skin Irritation and UVB Irradiation in 3D Reconstructed Human Skin Models
by
Francesca Truzzi, Camilla Tibaldi, Silvia Dilloo, Annalisa Saltari, Mitchell P. Levesque, Fabio Arcangeli, Alfredo Garzi, Giuseppe Ruggiero and Giovanni Dinelli
Pharmaceutics 2025, 17(4), 472; https://doi.org/10.3390/pharmaceutics17040472 (registering DOI) - 4 Apr 2025
Abstract
Background/Objectives: There is a great demand for novel, multipurpose, natural skin-care products in the global skin repair and sun protection markets. Within this framework, the potential benefits of topical Vitamin D2 (VD2) administration in combination with silver nanoparticles (AgNPs) were examined. Methods
[...] Read more.
Background/Objectives: There is a great demand for novel, multipurpose, natural skin-care products in the global skin repair and sun protection markets. Within this framework, the potential benefits of topical Vitamin D2 (VD2) administration in combination with silver nanoparticles (AgNPs) were examined. Methods: Evaluating the efficacy of the VD2+AgNP cream in wound healing, skin irritation and UVB irradiation protection necessitated preclinical testing using reconstructed human skin equivalent models (prepared from human foreskins) containing both a fully stratified epidermal layer and underlying dermis. Results: Application of the cream significantly improved wound healing by stimulating keratinocyte re-epithelialization and dermal fibroblast migration in models subjected to full-thickness (scratch and biopsy punch) wounds, compared to untreated models. The VD2+AgNP cream, administered prior to the induction of skin irritation by 5% sodium dodecyl sulfate (SDS) afforded protection by ameliorating cell viability epidermal thickness and interleukin-1alpha levels. UVB exposure (50 mJ/cm2) significantly reduced cell viability and epidermal thickness (associated with increased epidermal breakage), as well as basal layer Ki67 and supra-basal layer involucrin expression, compared to the CTRL sham-irradiated models. The cream administered prior to UVB irradiation (protective capacity) showed greater efficacy in minimizing epidermal damage. This was reflected by significantly higher Ki67 and involucrin expression, as well as lower epidermal breakage, compared to models where the cream was applied following UVB irradiation (curative capacity). Conclusions: The VD2+AgNP cream shows multipurpose potential in skin protection. The underlying molecular mechanisms remain to be investigated.
Full article
(This article belongs to the Special Issue Regenerative Biomaterials with Plant Derivatives for Skin Wound Therapy)
►▼
Show Figures

Graphical abstract
Open AccessReview
Targeting Regulatory Noncoding RNAs in Human Cancer: The State of the Art in Clinical Trials
by
Roberto Piergentili and Stefano Sechi
Pharmaceutics 2025, 17(4), 471; https://doi.org/10.3390/pharmaceutics17040471 - 4 Apr 2025
Abstract
Noncoding RNAs (ncRNAs) are a heterogeneous group of RNA molecules whose classification is mainly based on arbitrary criteria such as the molecule length, secondary structures, and cellular functions. A large fraction of these ncRNAs play a regulatory role regarding messenger RNAs (mRNAs) or
[...] Read more.
Noncoding RNAs (ncRNAs) are a heterogeneous group of RNA molecules whose classification is mainly based on arbitrary criteria such as the molecule length, secondary structures, and cellular functions. A large fraction of these ncRNAs play a regulatory role regarding messenger RNAs (mRNAs) or other ncRNAs, creating an intracellular network of cross-interactions that allow the fine and complex regulation of gene expression. Altering the balance between these interactions may be sufficient to cause a transition from health to disease and vice versa. This leads to the possibility of intervening in these mechanisms to re-establish health in patients. The regulatory role of ncRNAs is associated with all cancer hallmarks, such as proliferation, apoptosis, invasion, metastasis, and genomic instability. Based on the function performed in carcinogenesis, ncRNAs may behave either as oncogenes or tumor suppressors. However, this distinction is not rigid; some ncRNAs can fall into both classes depending on the tissue considered or the target molecule. Furthermore, some of them are also involved in regulating the response to traditional cancer-therapeutic approaches. In general, the regulation of molecular mechanisms by ncRNAs is very complex and still largely unclear, but it has enormous potential both for the development of new therapies, especially in cases where traditional methods fail, and for their use as novel and more efficient biomarkers. Overall, this review will provide a brief overview of ncRNAs in human cancer biology, with a specific focus on describing the most recent ongoing clinical trials (CT) in which ncRNAs have been tested for their potential as therapeutic agents or evaluated as biomarkers.
Full article
(This article belongs to the Special Issue mRNA Therapeutics for Cancer Treatment)
Open AccessReview
Modified-Release Pulmonary Delivery Systems for Labile Bioactives: Design, Development, and Applications
by
Shivani Nana, Mershen Govender and Yahya E. Choonara
Pharmaceutics 2025, 17(4), 470; https://doi.org/10.3390/pharmaceutics17040470 - 3 Apr 2025
Abstract
Pulmonary delivery of bioactives has shown to be a promising route for the treatment of respiratory conditions, however, numerous physiological barriers, such as mucociliary clearance and immune responses, pose significant hurdles to treatment efficacy. These barriers specifically affect labile bioactives such as mRNA,
[...] Read more.
Pulmonary delivery of bioactives has shown to be a promising route for the treatment of respiratory conditions, however, numerous physiological barriers, such as mucociliary clearance and immune responses, pose significant hurdles to treatment efficacy. These barriers specifically affect labile bioactives such as mRNA, peptides, proteins, and probiotics, which are susceptible to degradation due to the prevailing conditions. Various drug delivery platforms have been developed to address these challenges, including, among others, polymeric nanoparticles, micelles, liposomes, and solid lipid nanoparticles that encapsulate and protect the labile bioactives during formulation and administration, enabling improved bioavailability, sustained release, and enhanced formulation stability, while further modification of these platforms allows for targeted drug delivery. This review explores the advanced drug delivery systems that have been designed to protect and release labile active agents in a controlled and targeted manner to the lung, with a specific focus provided on the physiological barriers to effective pulmonary delivery and the formulation considerations to overcome these challenges. The outlook of this pertinent field of study has additionally been provided, highlighting the significant potential of the pulmonary delivery of labile bioactive agents for the prevention and treatment of a variety of respiratory ailments.
Full article
(This article belongs to the Special Issue Application of Nanomaterials in Pulmonary Drug Delivery)
Open AccessArticle
Targeted Delivery of Chlorin-e6-Loaded Carbon Nanotube-Based Nanobiocomposite to Cancer Stem Cells for Enhanced Photodynamic Therapy
by
Prabhavathi Sundaram, Sathish Sundar Dhilip Kumar and Heidi Abrahamse
Pharmaceutics 2025, 17(4), 469; https://doi.org/10.3390/pharmaceutics17040469 - 3 Apr 2025
Abstract
Background: Globally, colorectal cancer (CRC) is the third-most diagnosed cancer among males and the second-most diagnosed cancer among females. In cancer, stem cells are a subset of neoplastic cells capable of tumorigenesis and exhibit properties like normal stem cells. Moreover, they are resistant
[...] Read more.
Background: Globally, colorectal cancer (CRC) is the third-most diagnosed cancer among males and the second-most diagnosed cancer among females. In cancer, stem cells are a subset of neoplastic cells capable of tumorigenesis and exhibit properties like normal stem cells. Moreover, they are resistant to conventional cancer treatments and can repopulate the tumor following treatment. Cancer cells are stimulated to undergo apoptosis by photodynamic therapy (PDT), which involves a light source, a photosensitizer, and reactive oxygen species. Methods: In this study, colon cancer stem cells were isolated from colon cancer cells and characterized using flow cytometry and immunofluorescence techniques. To treat colon cancer stem cells (CCSCs), single-walled carbon nanotubes (SWCNTs) were coupled with hyaluronic acid (HA) and loaded with chlorin-e6 (Ce6). Nanobiocomposite toxicity was assessed using CCSCs with two fluences of 5 J/cm2 and 10 J/cm2. The cellular changes were observed at 24 and 48 h using microscopy, Results: LDH cytotoxicity assay, and cell death induction by annexin propidium iodide assay. An intracellular analysis of reactive oxygen species (ROS) detected oxidative stress within CCSCs. Conclusions: Overall, the results showed that the newly synthesized nanobiocomposite enhanced the ability of PDT to act as a photosensitizer carrier and induced cell death in CCSCs.
Full article
(This article belongs to the Special Issue Functional Nanomaterials for Drug Delivery in Photodynamic Therapy)
►▼
Show Figures

Figure 1
Open AccessArticle
C3-Liposome Delivery of MUC1 Peptide and TLR Agonists Enhances Adaptive Immunity and Results in Sex-Based Tumor Growth Differences
by
Shahab Soltani, Ameneh Arabi, Kristine Mann, Austin Hess, Holly A. Martinson and Max Kullberg
Pharmaceutics 2025, 17(4), 468; https://doi.org/10.3390/pharmaceutics17040468 - 3 Apr 2025
Abstract
Background: Mucin-1 (MUC1) is a glycoprotein that is hypoglycosylated and overexpressed in most adenocarcinomas, making it a promising target for cancer vaccines. Our group previously demonstrated that C3 (OPSS)-liposomes enhance antigen uptake by antigen-presenting cells (APCs) via the complement C3 pathway and,
[...] Read more.
Background: Mucin-1 (MUC1) is a glycoprotein that is hypoglycosylated and overexpressed in most adenocarcinomas, making it a promising target for cancer vaccines. Our group previously demonstrated that C3 (OPSS)-liposomes enhance antigen uptake by antigen-presenting cells (APCs) via the complement C3 pathway and, when combined with toll-like receptor (TLR) agonists, reduce tumor growth in murine cancer models. Methods: In the present study, we evaluate the immunogenicity of MUC1 peptide vaccines encapsulated in C3-liposomes, with and without TLR agonists, using MUC1-tolerant transgenic mice challenged with Lewis lung carcinoma (LLC.MUC1) cells. To assess vaccine effectiveness, tumor volumes were measured, and flow cytometry and ELISA and ELISPOT assays were used to assess the immune response. Results: Both male and female C57BL/6 transgenic mice vaccinated with MUC1 C3-liposomes developed significantly smaller tumors than those vaccinated with free MUC1 peptide or PBS. Notably, a sex-dependent response emerged in mice vaccinated with MUC1 C3-liposomes with TLR agonists (TLR4, TLR7/8, and TLR9); male mice exhibited greater tumor suppression than females. Flow cytometry analysis revealed that female mice had significantly higher levels of CD11b+, LY6C+, and LY6G+ MDSC cells, suggesting a potential mechanism for the sex difference. Additionally, MUC1 C3-liposome vaccination elicited robust adaptive immune responses, including significantly higher levels of IFN-γ-producing T cells and MUC1-specific IgG antibodies compared to non-encapsulated MUC1 or TLR adjuvant-only formulations. Conclusions: These findings underscore the potential of C3-liposome-based antigen vaccines to enhance anti-tumor immunity and highlight the impact of sex differences in vaccine efficacy.
Full article
(This article belongs to the Special Issue Lipid Nanostructures as Drug Carriers for Cancer Therapy)
►▼
Show Figures

Figure 1
Open AccessArticle
Polymer-Functionalized Magnetic Nanoparticles for Targeted Quercetin Delivery: A Potential Strategy for Colon Cancer Treatment
by
Júlia Borges de Macedo, Julia Narayana Schoroeder Bueno, Carla Cristine Kanunfre, José Ricardo de Arruda Miranda, Andris Figueiroa Bakuzis and Priscileila Colerato Ferrari
Pharmaceutics 2025, 17(4), 467; https://doi.org/10.3390/pharmaceutics17040467 - 3 Apr 2025
Abstract
Background/Objectives: Nanoparticle-based drug delivery systems improve pharmacokinetic aspects, including controlled release and drug targeting, increasing therapeutic efficacy, and reducing toxicity in conventional colon cancer treatment. The superparamagnetism of magnetic nanoparticles (MNP) appears to be a potential alternative for magnetothermal therapy, inducing tumor
[...] Read more.
Background/Objectives: Nanoparticle-based drug delivery systems improve pharmacokinetic aspects, including controlled release and drug targeting, increasing therapeutic efficacy, and reducing toxicity in conventional colon cancer treatment. The superparamagnetism of magnetic nanoparticles (MNP) appears to be a potential alternative for magnetothermal therapy, inducing tumor cell death by an external magnetic field. Therefore, this study aimed to develop chitosan (CS) and folate-chitosan (FA-CS)-coated MNP to improve the stability and targeting of the system for quercetin (Q) delivery. Methods: After FA-CS synthesis and 32 factorial design, polymer-functionalized MNPs were produced for quercetin loading, characterized, and evaluated by drug dissolution and cytotoxicity assay. Results: The factorial design indicated the positive influence of CS on MNPs’ Zeta potential, followed by the CS–temperature interaction. Optimized formulations had hydrodynamic diameters of 122.32 ± 8.56 nm, Zeta potentials of +30.78 ± 0.8 mV, and loading efficiencies of 80.45% (MNP-CS-Q) and 54.4% (MNP-FA-CS-Q). The 24 h drug release was controlled in MNP-CS-Q (up to 6.4%) and MNP-FA-CS-Q (up to 7.7%) in a simulated tumor medium, with Fickian diffusion release mechanism correlated to the Korsmeyer–Peppas model (R > 0.99). The cytotoxicity assay in HCT-116 showed a higher (p < 0.001) dose-dependent antitumor effect of quercetin-loaded MNP compared to free drug, with IC50s of 1.46 (MNP-CS) and 1.30 µg·mL−1 (MNP-FA-CS). Conclusions: Therefore, this study contributes to the development of biomedical nanotechnology and the magnetic debate by highlighting the antitumor potential of quercetin magnetic nanoparticles. The experimental design allows the discussion of critical manufacturing variables and the determination of optimal parameters for the formulations.
Full article
(This article belongs to the Special Issue Advancing Cancer Therapy: Magnetic Nanoparticles for Enhanced Targeted Drug Delivery)
►▼
Show Figures

Graphical abstract
Open AccessArticle
Optimization, In Vitro, and In Silico Characterization of Theophylline Inhalable Powder Using Raffinose-Amino Acid Combination as Fine Co-Spray-Dried Carriers
by
Petra Party, Lomass Soliman, Attila Nagy, Árpád Farkas and Rita Ambrus
Pharmaceutics 2025, 17(4), 466; https://doi.org/10.3390/pharmaceutics17040466 - 3 Apr 2025
Abstract
Background/Objectives: Dry powder inhalation is an attractive research area for development. Therefore, this work aimed to develop inhalable co-spray-dried theophylline (TN) microparticles, utilizing raffinose-amino acid fine carriers intended for asthma therapy. The study addressed enhancing TN’s physicochemical and aerodynamic properties to ensure
[...] Read more.
Background/Objectives: Dry powder inhalation is an attractive research area for development. Therefore, this work aimed to develop inhalable co-spray-dried theophylline (TN) microparticles, utilizing raffinose-amino acid fine carriers intended for asthma therapy. The study addressed enhancing TN’s physicochemical and aerodynamic properties to ensure efficient lung deposition. Methods: The process involves spray-drying each formulation’s solution using a mini spray drier. A rigorous assessment was conducted on particle size distribution, structural and thermal analysis, morphology study, in vitro and in silico aerodynamic investigation, and aerodynamic particle counter in addition to the solubility, in vitro dissolution, and diffusion of TN. Results: The carriers containing leucine and glycine revealed superior characteristics (mass median aerodynamic diameter (MMAD): 4.6–5 µm, fine particle fraction (FPF): 30.6–35.1%, and amorphous spherical structure) as candidates for further development of TN-DPIs, while arginine was excluded due to intensive aggregation and hygroscopicity, which led to poor aerodynamic performance. TN co-spray-dried samples demonstrated fine micronized particles (D [0.5]: 3.99–5.96 µm) with predominantly amorphous structure (crystallinity index: 24.1–45.2%) and significant solubility enhancement (~19-fold). Formulations containing leucine and leucine-glycine revealed the highest FPF (45.7–47.8%) and in silico lung deposition (39.3–40.1%), rapid in vitro drug release (~100% within 10 min), and improved in vitro diffusion (2.29–2.43-fold), respectively. Moreover, the aerodynamic counter confirmed the development of fine microparticles (mean number particle size = 2.3–2.02 µm). Conclusions: This innovative formulation possesses enhanced physicochemical, morphological, and aerodynamic characteristics of low-dose TN for local asthma treatment and could be applied as a promising carrier for dry powder inhaler development.
Full article
(This article belongs to the Special Issue Drug Product Performance: Bioavailability, Relative Bioavailability and Bioequivalence, 2nd Edition)
►▼
Show Figures

Graphical abstract
Open AccessEditorial
Transport of Drugs Through Biological Barriers—An Asset or Risk
by
Anna Weronika Sobańska
Pharmaceutics 2025, 17(4), 465; https://doi.org/10.3390/pharmaceutics17040465 - 3 Apr 2025
Abstract
Biological barriers are both cellular and enzymatic interfaces between different compartments of a living organism [...]
Full article
(This article belongs to the Special Issue Transport of Drugs through Biological Barriers—an Asset or Risk)
Open AccessReview
Vesicular Carriers for Phytochemical Delivery: A Comprehensive Review of Techniques and Applications
by
Shery Jacob, Fathima Sheik Kather, Sai H. S. Boddu, Rekha Rao and Anroop B. Nair
Pharmaceutics 2025, 17(4), 464; https://doi.org/10.3390/pharmaceutics17040464 - 2 Apr 2025
Abstract
Natural substances, especially those derived from plants, exhibit a diverse range of therapeutic benefits, such as antioxidant, anti-inflammatory, anticancer, and antimicrobial effects. Nevertheless, their use in clinical settings is frequently impeded by inadequate solubility, limited bioavailability, and instability. Nanovesicular carriers, such as liposomes,
[...] Read more.
Natural substances, especially those derived from plants, exhibit a diverse range of therapeutic benefits, such as antioxidant, anti-inflammatory, anticancer, and antimicrobial effects. Nevertheless, their use in clinical settings is frequently impeded by inadequate solubility, limited bioavailability, and instability. Nanovesicular carriers, such as liposomes, niosomes, ethosomes, transferosomes, transethosomes, and cubosomes, have emerged as innovative phytochemical delivery systems to address these limitations. This review highlights recent developments in vesicular nanocarriers for phytochemical delivery, emphasizing preparation techniques, composition, therapeutic applications, and the future potential of these systems. Phytosomes, along with their key advantages and various preparation techniques, are extensively described. Various in vitro and in vivo characterization techniques utilized for evaluating these nanovesicular carriers are summarized. Completed clinical trials and patents granted for nanovesicles encapsulating phytochemicals designed for systemic delivery are tabulated. Phytochemical delivery via vesicular carriers faces challenges such as low stability, limited active loading, scalability issues, and high production costs. Additionally, immune clearance and regulatory hurdles hinder clinical application, requiring improved carrier design and formulation techniques.
Full article
(This article belongs to the Special Issue Novel Drug Delivery Systems for Natural Extracts)
►▼
Show Figures

Figure 1
Open AccessArticle
The Incorporation of CBD into Biodegradable DL-Lactide/Glycolide Copolymers Creates a Persistent Antibacterial Environment: An In Vitro Study on Streptococcus mutans and Staphylococcus aureus
by
Ronit Vogt Sionov, Ahmad Siag, Emma Theresa Mersini, Natalya M. Kogan, Tatiana Alkhazov, Igor Koman, Praveen Rowlo, Vitaly Gutkin, Menachem Gross and Doron Steinberg
Pharmaceutics 2025, 17(4), 463; https://doi.org/10.3390/pharmaceutics17040463 - 2 Apr 2025
Abstract
Background: Cannabidiol (CBD) is a natural compound from the Cannabis sativa L. plant, which has anti-inflammatory, anti-nociceptive, neuroprotective, and antibacterial activities. Objective: The aim of this study was to develop a sustained-release device of CBD that can provide an antibacterial effect
[...] Read more.
Background: Cannabidiol (CBD) is a natural compound from the Cannabis sativa L. plant, which has anti-inflammatory, anti-nociceptive, neuroprotective, and antibacterial activities. Objective: The aim of this study was to develop a sustained-release device of CBD that can provide an antibacterial effect against the Gram-positive bacteria Streptococcus mutans and Staphylococcus aureus for extended periods of time. Methods: CBD was incorporated into the biodegradable PURASORB 5010 or PURASORB 7510 DL-lactide/glycolide polymers using either dimethylsulfoxide (DMSO) or acetone as the solvent, and the dried polymer scaffolds were exposed daily to a fresh culture of bacteria. The bacterial growth was determined daily by optical density, and the metabolic activity of biofilms was determined using the MTT assay. Biofilm formation on the polymer scaffolds was visualized by HR-SEM. Its anti-inflammatory effect was determined by measuring the IL-6 release from LPS-stimulated RAW 264.7 macrophages by ELISA. Cell cytotoxicity on normal Vero epithelial cells was determined by the MTT assay. The daily release of CBD was determined by gas chromatography–mass spectrometry (GC-MS). Results: PURASORB 5010/CBD scaffolds had antibacterial activity against S. mutans UA159, S. aureus ATCC25923, and a clinical isolate of a multidrug-resistant S. aureus (MDRSA CI-M) strain for the tested period of up to 17 days. PURASORB 7510/CBD scaffolds also had antibacterial activity, but overall, it was less effective than PURASORB 5010/CBD over time. The addition of PEG400 to the copolymers significantly increased the antibacterial activity of PURASORB 7510/CBD but not of PURASORB 5010/CBD. The daily release of CBD from the polymer scaffolds was sufficient to reduce the LPS-induced IL-6 secretion from RAW 264.7 macrophages, and importantly, it was not cytotoxic to either RAW 264.7 macrophages or Vero epithelial cells. The daily release of CBD was found to be between 1.12 and 9.43 µg/mL, which is far below the cytotoxic dose of 25 µg/mL. Conclusions: The incorporation of CBD into the biodegradable PURASORB 5010 can be used to prepare sustained-release devices for medical purposes where combined antibacterial and anti-inflammatory activities are desirable.
Full article
(This article belongs to the Special Issue Natural Products for Antimicrobial and Drug Delivery: A Look into the Future)
►▼
Show Figures
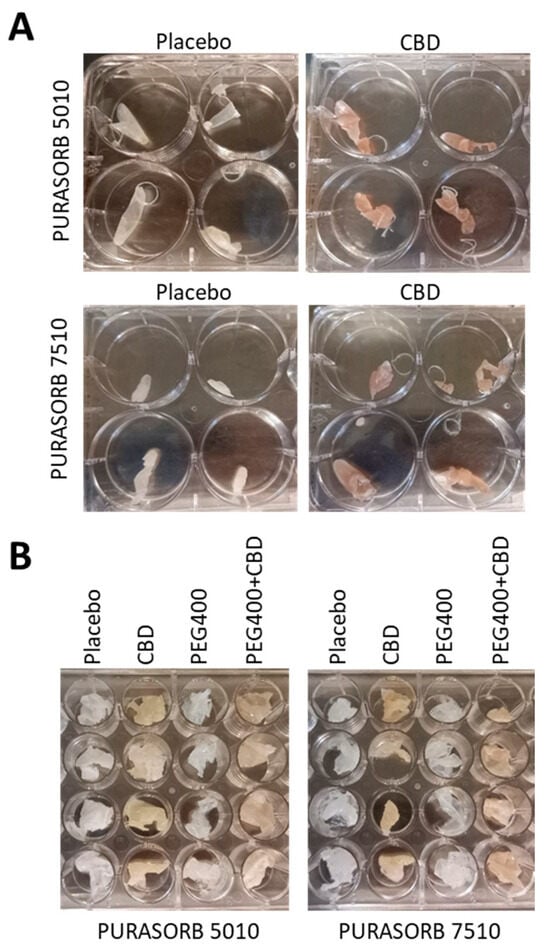
Figure 1
Open AccessArticle
Development and Characterization of a Primary Ciliated Porcine Airway Model for the Evaluation of In Vitro Mucociliary Clearance and Mucosal Drug Delivery
by
Janik Martin, Veronika Neubauer, Rebecca Rittersberger, Simon Treitler, Patrick Kopp, Cemre Günday, Iman Shrimo, Annabelle Dabbars, Frank Rosenau, Akif Emre Türeli, Nazende Günday-Türeli, Oliver Haedicke-Peters and Katharina Schindowski
Pharmaceutics 2025, 17(4), 462; https://doi.org/10.3390/pharmaceutics17040462 - 2 Apr 2025
Abstract
Background/Objectives: In vitro models play a crucial role in preclinical respiratory research, enabling the testing and screening of mucosal formulations, dosage forms, and inhaled drugs. Mucociliary clearance (MCC) is an essential defense mechanism in mucosal drug delivery but is often impaired in
[...] Read more.
Background/Objectives: In vitro models play a crucial role in preclinical respiratory research, enabling the testing and screening of mucosal formulations, dosage forms, and inhaled drugs. Mucociliary clearance (MCC) is an essential defense mechanism in mucosal drug delivery but is often impaired in respiratory diseases. Despite its importance, standardized in vitro MCC assays are rarely reported. Furthermore, many published methods primarily measure cilia beat frequency (CBF), which requires high-speed cameras that are not accessible to all laboratories. Therefore, this study aimed to develop a physiologically relevant, differentiated in vitro model of the respiratory epithelium that incorporates both beating cilia and functional MCC. We chose porcine airway mucosa as an alternative to human tissue due to ethical considerations and limited availability. The established model is designed to provide a reproducible and accessible method for a broad range of research laboratories. Methods: The previously published tracheal mucosal primary cell (TMPC DS) model, derived from porcine tissue, lacked the presence of beating cilia, which are crucial for effective MCC analysis. For accurate MCC assessment, beating cilia are essential as they play a key role in mucus clearance. To address this limitation, the here-described ciliated tracheal mucosal primary cell (cTMPC) model was developed. cTMPCs were isolated from porcine tissue and cultured under air–liquid interface (ALI) conditions for 21 days to promote differentiation. This model was evaluated for cell morphology, tight junction formation, ciliated and mucus-producing cells, barrier function, gene expression, and tracer/IgG transport. MCC and the model’s suitability for standardized MCC assays were assessed using an inverted microscope. In contrast to the TMPC DS model, which lacked beating cilia and thus could not support MCC analysis, the cTMPC model allows for comprehensive MCC studies. Results: The developed differentiated in vitro model demonstrated key structural and functional features of the respiratory epithelium, including well-differentiated cell morphology, tight junction integrity, ciliated and mucus-producing cells, and effective barrier function. Functional MCC was observed, confirming the model’s potential for standardized clearance assays. Conclusions: This differentiated in vitro model closely replicates the structural and functional characteristics of in vivo airways. It provides a valuable platform for studying mucociliary clearance, toxicology, drug uptake, and evaluating mucosal formulations and dosage forms in respiratory research.
Full article
(This article belongs to the Special Issue Advances in Drug Delivery Systems for Transmucosal Routes Applied in the Treatment of Infectious and Chronic Degenerative Diseases)
►▼
Show Figures
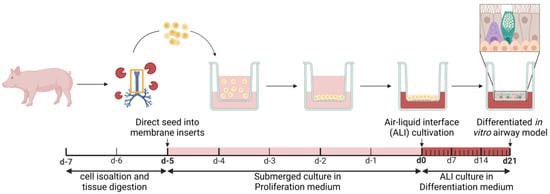
Figure 1

Journal Menu
► ▼ Journal Menu-
- Pharmaceutics Home
- Aims & Scope
- Editorial Board
- Reviewer Board
- Topical Advisory Panel
- Instructions for Authors
- Special Issues
- Topics
- Sections & Collections
- Article Processing Charge
- Indexing & Archiving
- Editor’s Choice Articles
- Most Cited & Viewed
- Journal Statistics
- Journal History
- Journal Awards
- Society Collaborations
- Conferences
- Editorial Office
Journal Browser
► ▼ Journal BrowserHighly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Cancers, Cells, JCM, Radiation, Pharmaceutics, Applied Sciences, Nanomaterials, Current Oncology
Innovative Radiation Therapies
Topic Editors: Gérard Baldacchino, Eric Deutsch, Marie Dutreix, Sandrine Lacombe, Erika Porcel, Charlotte Robert, Emmanuelle Bourneuf, João Santos Sousa, Aurélien de la LandeDeadline: 30 April 2025
Topic in
Chemistry, Materials, Molecules, Polymers, Pharmaceutics
Advanced Biomaterials: Processing and Applications
Topic Editors: Vincenzo Guarino, Roberto De Santis, Ugo D'AmoraDeadline: 31 May 2025
Topic in
Biomolecules, IJMS, Molecules, Pharmaceutics
Advances in Diagnostics, Brain Delivery Systems and Therapeutics of Neurodegenerative Disease
Topic Editors: Ashok Iyaswamy, Chuanbin Yang, Abhimanyu ThakurDeadline: 11 June 2025
Topic in
Antioxidants, Biomolecules, JCDD, Metabolites, Neurology International, Pharmaceutics
Tissue-Specific, Disease-Signatured Macrophages in Control of Redox and Antioxidation in Metabolic Diseases
Topic Editors: Xiangwei Xiao, Yingmei Feng, Zhiyong LeiDeadline: 5 July 2025

Conferences
Special Issues
Special Issue in
Pharmaceutics
Recent Progress in Microencapsulation and Nanoencapsulation for Pharmaceutical Applications
Guest Editor: Ljiljana ĐekićDeadline: 10 April 2025
Special Issue in
Pharmaceutics
Functional Nanomaterials for Drug Delivery in Photodynamic Therapy
Guest Editors: Enrico Caruso, Miryam Chiara MalacarneDeadline: 10 April 2025
Special Issue in
Pharmaceutics
Nanofibrous Scaffolds Application in Biomedicine
Guest Editors: Paola Nitti, Marta Madaghiele, Christian DemitriDeadline: 10 April 2025
Special Issue in
Pharmaceutics
Novel Drug Delivery Systems: Magnetic Gels
Guest Editor: Carlos O. AmorimDeadline: 10 April 2025
Topical Collections
Topical Collection in
Pharmaceutics
Advanced Pharmaceutical Science and Technology in Korea
Collection Editors: Hyo-Kyung Han, Beom-Jin Lee
Topical Collection in
Pharmaceutics
Pharmaceutical Sciences in Canada
Collection Editors: Kishor Wasan, Ildiko Badea
Topical Collection in
Pharmaceutics
Advanced Pharmaceutical Science and Technology in Japan
Collection Editors: Yusuke Sato, Yoshinobu Takakura
Topical Collection in
Pharmaceutics
Advanced Pharmaceutical Research in the Czech Republic
Collection Editors: Jarmila Zbytovska, Jan Gajdziok, Jakub Vysloužil








