Journal Description
Vaccines
Vaccines
is an international, peer-reviewed, open access journal published monthly online by MDPI.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, SCIE (Web of Science), PubMed, PMC, Embase, CAPlus / SciFinder, and other databases.
- Journal Rank: JCR - Q1 (Immunology) / CiteScore - Q1 (Pharmacology (medical))
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 18.6 days after submission; acceptance to publication is undertaken in 3.3 days (median values for papers published in this journal in the second half of 2024).
- Recognition of Reviewers: reviewers who provide timely, thorough peer-review reports receive vouchers entitling them to a discount on the APC of their next publication in any MDPI journal, in appreciation of the work done.
Impact Factor:
5.2 (2023);
5-Year Impact Factor:
4.9 (2023)
Latest Articles
No Increased Risk of Autoimmune Diseases Following HPV Vaccination: A Systematic Review and Meta-Analysis
Vaccines 2025, 13(4), 391; https://doi.org/10.3390/vaccines13040391 (registering DOI) - 7 Apr 2025
Abstract
Background: HPV vaccination reduces the risk of anogenital warts, high-grade cervical intraepithelial neoplasia (CIN2+), and cervical cancer. To enhance immunogenicity, HPV vaccines include adjuvants such as toll-like receptor agonists, which may theoretically trigger autoimmune responses. However, existing data on this risk remain conflicting.
[...] Read more.
Background: HPV vaccination reduces the risk of anogenital warts, high-grade cervical intraepithelial neoplasia (CIN2+), and cervical cancer. To enhance immunogenicity, HPV vaccines include adjuvants such as toll-like receptor agonists, which may theoretically trigger autoimmune responses. However, existing data on this risk remain conflicting. This systematic review and meta-analysis assess the association between HPV vaccination and autoimmune disease onset in post-licensure controlled studies. Methods: A comprehensive literature search was conducted in Scopus, PubMed/MEDLINE, ScienceDirect, and the Cochrane Library from inception to June 2024, following PRISMA guidelines. The study protocol was registered in PROSPERO (CRD42024606834). Results: A total of 356 studies were identified, including cross-reference reviews. Fourteen met inclusion criteria for qualitative and quantitative analysis, encompassing 8,088,838 patients, of whom 2,041,865 received the HPV vaccine. Conclusions: This meta-analysis found no significant association between HPV vaccination and autoimmune disease development. However, further large-scale observational studies are needed, particularly among male recipients, as current evidence is predominantly based on female populations. Future research should also evaluate risks for specific autoimmune disorders to refine the vaccine’s safety profile.
Full article
(This article belongs to the Special Issue State-of-the-Art Vaccine Design)
►
Show Figures
Open AccessArticle
Vaccination Against RhoC in Prostate Cancer Patients Induces Potent and Long-Lasting CD4+ T Cell Responses with Cytolytic Potential in the Absence of Clinical Efficacy: A Randomized Phase II Trial
by
Sara Fresnillo Saló, Juliane Schuhmacher, Anne Rahbech, Sara Ram Pedersen, Tina Seremet, Valero Andreu Matillas, Anna Schöllhorn, Andreas Røder, Steffen Wad Jørgensen, Klaus Brasso, Cécile Gouttefangeas, Per thor Straten and on behalf of the RhoVac-002 Study Group
Vaccines 2025, 13(4), 390; https://doi.org/10.3390/vaccines13040390 (registering DOI) - 5 Apr 2025
Abstract
Background: A previous phase I/II study demonstrated potent and long-term immune responses in men with prostate cancer following vaccination with a 20mer synthetic peptide (RV001) derived from the Ras homolog gene family member C protein (RhoC). Moreover, a fraction of patients experienced
[...] Read more.
Background: A previous phase I/II study demonstrated potent and long-term immune responses in men with prostate cancer following vaccination with a 20mer synthetic peptide (RV001) derived from the Ras homolog gene family member C protein (RhoC). Moreover, a fraction of patients experienced prostate-specific antigen (PSA) responses, which prompted the initiation of a phase II double-blind randomized trial (NCT04114825). The primary endpoint was to study whether vaccination could postpone PSA progression. Furthermore, the study included an evaluation of vaccination-induced immune responses, and in-depth in vitro studies of RhoC-specific CD4+ T cell responses. Methods: Men with non-metastatic biochemical recurrence after either radical prostatectomy or radiation therapy were eligible for the study. Participants were randomized 1:1 to either subcutaneous injections of 0.1 mg/mL RV001 emulsified in Montanide ISA 51, or a placebo. Vaccinations were administered every 2 weeks for the first six times, then five times every 4 weeks for a total treatment time of 30 weeks. Blood samples were collected from a subset of patients (n = 38) over the course of vaccination, and peripheral blood mononuclear cells (PBMCs) isolated for immunological assessment of vaccine-induced immune responses. Experiments using PBMCs from a healthy donor and a patient were performed to study the phenotype and function of RV001-specific CD4+ T cells. Results: A total of 192 men entered the study. There was no difference in time to PSA doubling, with 7.5 versus 9.3 months, or in time to initiating further therapies, 11.2 versus 17.6 months for treatment and control groups, respectively. At long-term follow-up, 12.9% of the patients in the vaccination arm had developed metastasis compared to 12% in the placebo arm. No serious treatment-related side effects were observed, and treatment-related adverse events did not differ between groups. Immunological examinations in a subset of patients demonstrated that the vaccination induced potent, long-lasting CD4+ T cell responses capable of proliferation and cytokine production. RV001-specific CD4+ T cells were shown to mediate cytotoxicity against a RhoC-expressing cancer cell line in an HLA-class II-dependent manner. Conclusions: Men randomized to active treatment with RV001V demonstrated the induction of potent, functionally capable, anti RhoC-CD4+ T cell responses. However, there was no benefit in time to biochemical progression, and no difference in time to the initiation of second-line therapies.
Full article
(This article belongs to the Special Issue Analysis of Vaccine-Induced Adaptive Immune Responses)
►▼
Show Figures

Figure 1
Open AccessReview
The Application of mRNA Technology for Vaccine Production—Current State of Knowledge
by
Anna Paczkowska, Karolina Hoffmann, Agata Andrzejczak, Weronika Faustyna Pucek, Dorota Kopciuch, Wiesław Bryl, Elżbieta Nowakowska and Krzysztof Kus
Vaccines 2025, 13(4), 389; https://doi.org/10.3390/vaccines13040389 - 4 Apr 2025
Abstract
Over the past 20 years, intensive research has been conducted on the development of therapeutic mRNA, leading to numerous discoveries that have enabled its use in therapy. The main achievements in this field include increasing mRNA stability, reducing its immunogenicity (i.e., its ability
[...] Read more.
Over the past 20 years, intensive research has been conducted on the development of therapeutic mRNA, leading to numerous discoveries that have enabled its use in therapy. The main achievements in this field include increasing mRNA stability, reducing its immunogenicity (i.e., its ability to trigger an immune response), and solving the challenge of delivering mRNA into cells—all to achieve a therapeutic effect. The aim of this study was to review the scientific literature on the use of mRNA technology in the production of vaccines. Various methods of applying mRNA technology that could potentially be introduced into clinical practice in the future are described. A detailed analysis was conducted on the approved COVID-19 vaccines developed by Pfizer/BioNTech (New York, NY, USA) and Moderna (Kirkland, QC, Canada), as their introduction marked a groundbreaking moment in the advancement of mRNA technology. This study was based on the latest scientific literature from reputable publishers and medical databases such as PubMed and ClinicalTrials. In conclusion, mRNA technology is currently experiencing rapid development, significantly driven by the ongoing COVID-19 pandemic. The application of this technology holds great potential not only for vaccines against infectious diseases but also for cancer treatment. However, further research is necessary to facilitate its broader clinical implementation.
Full article
(This article belongs to the Special Issue Vaccine Development and Global Health)
Open AccessArticle
Routine Immunisation Coverage Shows Signs of Recovery at Global Level Postpandemic, but Important Declines Persist in About 20% of Countries
by
Beth Evans, Laurent Kaiser, Olivia Keiser and Thibaut Jombart
Vaccines 2025, 13(4), 388; https://doi.org/10.3390/vaccines13040388 (registering DOI) - 3 Apr 2025
Abstract
Background/Objectives: Routine immunisation (RI) coverage declines during the COVID-19 pandemic, from 2020 to 2022, are well-reported. With the declared end to the Public Health Emergency of International Concern in May 2023, and the cessation of most nonpharmaceutical interventions that were introduced to prevent
[...] Read more.
Background/Objectives: Routine immunisation (RI) coverage declines during the COVID-19 pandemic, from 2020 to 2022, are well-reported. With the declared end to the Public Health Emergency of International Concern in May 2023, and the cessation of most nonpharmaceutical interventions that were introduced to prevent or minimise COVID-19 spread, we (I) assess whether routine immunisation coverage has rebounded to the level of prepandemic trends and (II) seek to identify factors that help predict whether country performance has exceeded, maintained, or declined compared with expectations (based on time-series forecasting). Methods: We quantified global and country-level routine immunisation diphtheria–tetanus–pertussis (DTP) coverage trends postpandemic (2023) compared with prepandemic trends using time-series forecasting across 190 countries. We used discriminant analysis of principal components and random forests to identify relevant predictors of country-level coverage performance, including twenty-eight indicators of health system strength, health workforce, country income, pandemic containment, economic and health policies, and demographic aspects. Results: We show that mean global DTP third-dose coverage levels remained on average 2.7% [95% confidence intervals: 1.1–4.3%] lower than expected in 2023. However, once accounting for temporal demographic changes, we find that this translated to the total number of immunised children almost reverting to expected levels because of decreasing fertility reducing global-level immunisation target populations. At the country level, notable disruption remained in over thirty countries (16.8% of countries below expectations, 81.6% within expected ranges, and 1.6% above expectations). Neither predictive method performed well at identifying factors associated with coverage disruptions. Conclusions: Despite the end of COVID-19 pandemic measures, RI remains below expectations in about 20% of countries. No clear drivers of this continued disruption were identified. Further research is required to inform recovery efforts and prevent future epidemic and pandemic disruptions to routine health services.
Full article
(This article belongs to the Special Issue Immunization Strategies and Vaccine Uptake after the SARS-CoV-2 Pandemic)
►▼
Show Figures
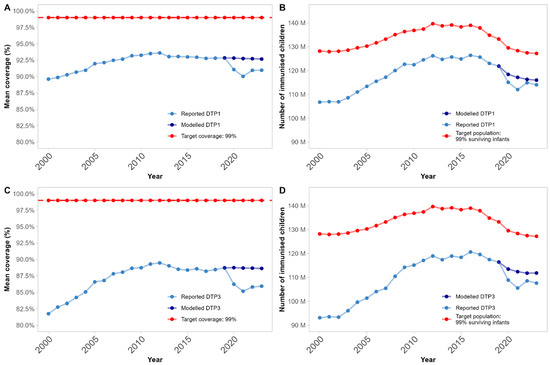
Figure 1
Open AccessArticle
Vaccination Coverage and Adherence to Scheduling in Children Aged 0 to 18 Months: Effects of COVID-19 and Age
by
Ubaldo Miranda-Soberón, Isabel Pino-Arana, Norma Pastor-Ramírez, Elena Figueroa-Cabezudo, Cyntia Zevallos-Parra and Gabriela Valencia-Borja
Vaccines 2025, 13(4), 387; https://doi.org/10.3390/vaccines13040387 - 3 Apr 2025
Abstract
►▼
Show Figures
Vaccination in Peru began 50 years ago as part of the Expanded Program on Immunization (EPI), which has proven effective in saving the lives of millions of children. This research aimed to determine the coverage and adherence to the vaccination schedule in children
[...] Read more.
Vaccination in Peru began 50 years ago as part of the Expanded Program on Immunization (EPI), which has proven effective in saving the lives of millions of children. This research aimed to determine the coverage and adherence to the vaccination schedule in children up to 18 months of age during the period 2018–2022, including the COVID-19 pandemic lockdown, in order to assess its influence. Materials and methods: This was a secondary source study based on the Demographic and Family Health Survey (ENDES) of Peru, including a sample of 82,702 male and female children whose caregivers presented vaccination cards. Coverage and adherence indicators were calculated, and differences were evaluated between the pre-confinement, absolute confinement, and relative confinement periods using a chi-square test. Results: For almost all vaccines, coverage decreased from 2018 to 2022 (from 82.46% to 80.16% on average, p < 0.001). Coverage also decreased as the scheduled age increased (0–2 months: median 93%, 7–18 months: median 63%; p < 0.001). Average adherence rates also declined over time (2018: 65.82% to 2022: 61.77%). The most affected vaccine was the yellow fever vaccine. Coverage did not reach protective population levels, while adherence has averaged 85.06% since 2018. Conclusions: COVID-19 negatively influenced compliance with the vaccination schedule and adherence.
Full article
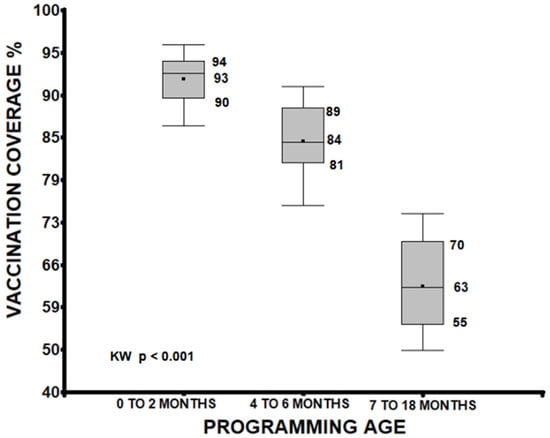
Figure 1
Open AccessArticle
Estimating the Public Health and Economic Impact of Annual mRNA COVID-19 Vaccination for Adults Aged 50 and Older in South Korea’s Endemic Era
by
Jaehee Jung, Dain Lee, Hee-Do Yang, Ah-Young Kim, Haeun Lee, Minkyoung Kang, Ekkehard Beck, Keya Joshi, Youngju Kang and Hye-Young Kang
Vaccines 2025, 13(4), 386; https://doi.org/10.3390/vaccines13040386 - 3 Apr 2025
Abstract
Background/Objectives: COVID-19 continues to challenge public health due to emerging variants. To mitigate this, the Korea Disease Control and Prevention Agency (KDCA) recommends annual COVID-19 vaccination, but uptake remains suboptimal. This study evaluates the public health and economic impact of annual mRNA COVID-19
[...] Read more.
Background/Objectives: COVID-19 continues to challenge public health due to emerging variants. To mitigate this, the Korea Disease Control and Prevention Agency (KDCA) recommends annual COVID-19 vaccination, but uptake remains suboptimal. This study evaluates the public health and economic impact of annual mRNA COVID-19 vaccination for adults aged 50 and older in South Korea during the 2024–2025 season, focusing on hospitalizations and costs. Methods: We estimated hospitalizations prevented by the mRNA-1273 XBB.1.5 containing vaccine by calculating symptomatic infection incidence rates, hospitalization rates among unvaccinated individuals, vaccine effectiveness (VE) against hospitalization, and vaccination rates. Incidence rates among the unvaccinated with an annual vaccine were derived by adjusting overall infection rates based on vaccination coverage and VE against COVID-19 hospitalization rates. Hospitalization costs were obtained from a real-world dataset, integrating the KDCA’s COVID-19 confirmed cases with National Health Insurance claims data. Comparative analyses between mRNA-1273 and BNT162b2 used published meta-analysis results. Results: Assuming vaccination rates remain consistent with the 2023–2024 season, mRNA-1273 is projected to prevent 37,200 hospitalizations and save USD 77.2 million in healthcare costs during the 2024–2025 season compared to no annual vaccination. Compared to BNT162b2, it is expected to prevent an additional 13,260 hospitalizations saving USD 27.5 million. If vaccination rates increased to match influenza, hospitalizations prevented by mRNA-1273 could rise to 79,800 with USD 164.2 million in healthcare savings compared to no annual vaccination. Conclusion: Annual mRNA COVID-19 vaccination with mRNA-1273 substantially reduces hospitalizations and healthcare costs. Increasing vaccination rates are essential to maximize public health benefits.
Full article
(This article belongs to the Special Issue Promoting Research, Development and Access to Vaccines to Address Global Inequities)
►▼
Show Figures
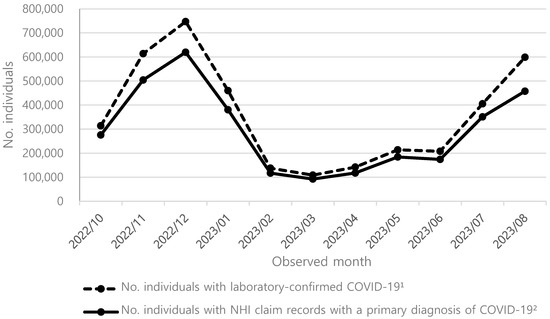
Figure 1
Open AccessBrief Report
Entry Efficiency, Protease Dependence, and Antibody-Mediated Neutralization of SARS-CoV-2 Sublineages KP.3.1.1 and XEC
by
Prerna Arora, Amy Kempf, Inga Nehlmeier, Sebastian R. Schulz, Hans-Martin Jäck, Markus Hoffmann and Stefan Pöhlmann
Vaccines 2025, 13(4), 385; https://doi.org/10.3390/vaccines13040385 - 3 Apr 2025
Abstract
Background: The SARS-CoV-2 variants KP.3.1.1 and XEC currently dominate the COVID-19 epidemic. However, their cell tropism, proteolytic processing, and susceptibility to neutralization by monoclonal antibodies remain incompletely characterized. Methods: We employed pseudotyped viruses to assess the entry efficiency of KP.3.1.1 and XEC in
[...] Read more.
Background: The SARS-CoV-2 variants KP.3.1.1 and XEC currently dominate the COVID-19 epidemic. However, their cell tropism, proteolytic processing, and susceptibility to neutralization by monoclonal antibodies remain incompletely characterized. Methods: We employed pseudotyped viruses to assess the entry efficiency of KP.3.1.1 and XEC in various cell lines, their dependence on TMPRSS2 for lung cell entry, and their ability to use ACE2 for infection. Additionally, we evaluated their susceptibility to neutralization by monoclonal antibodies BD55-4637 and BD55-5514. Results: KP.3.1.1 and XEC entered cell lines with similar efficiency as the parental JN.1 lineage and utilized TMPRSS2 for Calu-3 lung cell entry. Unlike JN.1, KP.3.1.1 and XEC failed to efficiently use murine ACE2 for cell entry. Both variants were effectively neutralized by the monoclonal antibodies BD55-4637 and BD55-5514, suggesting therapeutic potential. Conclusions: Our findings demonstrate that JN.1, KP.3.1.1, and XEC, like their predecessor BA.2.86, rely on TMPRSS2 for lung cell entry and remain sensitive to certain neutralizing monoclonal antibodies. However, these variants differ in their ability to utilize ACE2 species orthologs for cell entry.
Full article
(This article belongs to the Special Issue SARS-CoV-2 Variants, Vaccines, and Immune Responses)
►▼
Show Figures

Figure 1
Open AccessBrief Report
Evaluating Vaccination Status and Barriers in Children with Rheumatic Diseases
by
Shine Vazhappilly, Babatope O. Adebiyi, Racheal Githumbi, Nicole A. Johnson, Otto G. Vanderkooi and Heinrike Schmeling
Vaccines 2025, 13(4), 384; https://doi.org/10.3390/vaccines13040384 - 3 Apr 2025
Abstract
Background: This study aims to evaluate the vaccination status of children with rheumatic diseases (RD) compared to healthy controls (HC) and immunization barriers, as studies examining the vaccination status and factors promoting or hindering vaccination among children RD remain limited. Methods:
[...] Read more.
Background: This study aims to evaluate the vaccination status of children with rheumatic diseases (RD) compared to healthy controls (HC) and immunization barriers, as studies examining the vaccination status and factors promoting or hindering vaccination among children RD remain limited. Methods: A cross-sectional study was conducted on children with RD (in a rheumatology clinic) and HC (in a fracture clinic) at a tertiary care center in Canada. Demographics, diagnosis, treatments, and vaccine status were obtained from health records and a provincial electronic vaccine database. A patient/caregiver questionnaire was used to capture perceived immunization barriers, concerns, and satisfaction. Descriptive statistical methods were used for analysis. Results: The study involved 144 children with RD and 111 HC. Data from 94 children with RD and 86 HC, all lifelong Alberta residents, were analyzed for objective vaccination status. Most vaccines were received at rates of 80% or higher, except the influenza vaccine, which had the lowest adherence (34% in RD vs. 21% in HC). In 31% of RD children, vaccinations were withheld due to active disease, healthcare provider advice, or caregiver concerns about side effects. In 27% HC, vaccinations were withheld due to side effects. Both groups primarily relied on their family doctor for vaccination information, and 85% or more expressed satisfaction with the information received. Conclusions: Most children with RD and HC received recommended vaccines, but influenza vaccination gaps were identified. Knowledge about vaccine contraindications in RD is well understood, but perceived safety concerns limit vaccination completeness. Healthcare providers, especially family doctors, pediatricians, and rheumatologists, should be providing education resources for vaccines and be proactive in discussing the safety and necessity of vaccinations.
Full article
(This article belongs to the Special Issue Virus Pandemics and Vaccinations)
Open AccessArticle
A Phase 1/2 Randomized Study to Evaluate the Safety, Tolerability, and Immunogenicity of Nucleoside-Modified Messenger RNA Influenza Vaccines in Healthy Adults
by
Angela Branche, Mark J. Mulligan, Alok Maniar, Orlando Puente, Islamiat Oladipupo, Graham Crowther, Agnieszka M. Zareba, Zhuobiao Yi, Ingrid Scully, Emily Gomme, Kenneth Koury, Nicholas Kitchin, Pirada Suphaphiphat Allen, Annaliesa S. Anderson, Alejandra Gurtman and Kelly Lindert
Vaccines 2025, 13(4), 383; https://doi.org/10.3390/vaccines13040383 - 3 Apr 2025
Abstract
►▼
Show Figures
Background/Objectives: Circulating influenza strains antigenically differing from vaccine antigens increase disease burden by decreasing vaccine efficacy. Nucleoside-modified mRNA (modRNA) influenza vaccines may facilitate rapid production allowing later antigen selection and improved antigenic similarity compared to circulating strains. We studied different influenza modRNA vaccine
[...] Read more.
Background/Objectives: Circulating influenza strains antigenically differing from vaccine antigens increase disease burden by decreasing vaccine efficacy. Nucleoside-modified mRNA (modRNA) influenza vaccines may facilitate rapid production allowing later antigen selection and improved antigenic similarity compared to circulating strains. We studied different influenza modRNA vaccine (IRV) formulations and dose levels. Methods: This phase 1/2 randomized study evaluated IRV safety/tolerability and immunogenicity in healthy 18- through 85-year-olds. Based on safety and immunogenicity for different IRV doses, schedules, and valencies versus the quadrivalent influenza vaccine (QIV; Fluzone High-Dose Quadrivalent, Sanofi Pasteur) in phase 1 (65–85-year-olds), quadrivalent IRV (qIRV) was further evaluated in 65- through 85-year-olds and 18- through 64-year-olds in phase 2, leading to phase 3 dose selection. Results: Phase 1 (65–85-year-olds) safety/tolerability and immunogenicity findings supported qIRV 30-µg and 60-µg phase 2 assessment (18–85-year-olds, N = 610). qIRV was well tolerated. Injection site pain was the most frequently reported local reaction. Reactogenicity event incidences ≤ 7 days postvaccination for qIRV were generally higher versus QIV, observed more frequently in 18- through 64-year-olds than 65- through 85-year-olds, and showed dose-related trends (60 μg > 30 μg). qIRV and QIV adverse event profiles in 65- through 85-year-olds were similar. There were higher postvaccination hemagglutination inhibition assay geometric mean titers and fold rises and seroconversion rates observed with qIRV versus QIV for A strains, with no consistent pattern for B strains. Cell-mediated immune responses to qIRV by Day 7 showed overall higher T-cell responses against all strains versus QIV. Antibody and cell-mediated immune responses showed comparable trends across qIRV doses in 18- through 85-year-olds; a dose-related pattern was observed in 65- through 85-year-olds (60 μg > 30 μg). Conclusions: Phase 3 investigations of qIRV 60 µg in older adults and qIRV 30 µg in younger adults are warranted (ClinicalTrials.gov Identifier: NCT05052697).
Full article

Figure 1
Open AccessBrief Report
Timing of Measles, Mumps, and Rubella Vaccination: Secondary Outcomes from an Immunological Survey
by
Jana Zibolenová, Romana Ulbrichtová, Eva Malobická, Martin Novák, Tibor Baška, Lucia Časnocha Lúčanová, Ján Mikas, Adriana Mečochová and Henrieta Hudečková
Vaccines 2025, 13(4), 382; https://doi.org/10.3390/vaccines13040382 - 3 Apr 2025
Abstract
Background/Objectives: This study analyzed data on the actual timing of the first and second doses of the Measles, Mumps, and Rubella (MMR) vaccination in Slovakia according to the vaccination schedule. Methods: Histograms were constructed using immunological survey data on MMR vaccination conducted in
[...] Read more.
Background/Objectives: This study analyzed data on the actual timing of the first and second doses of the Measles, Mumps, and Rubella (MMR) vaccination in Slovakia according to the vaccination schedule. Methods: Histograms were constructed using immunological survey data on MMR vaccination conducted in Slovakia in 2018. Results: For the first dose (2560 individuals), 83.4% of them were vaccinated timely (15th–18th month, mostly in the 16th month), while 13.8% of them were delayed. For the second dose (1061 individuals), 72.7% of vaccinations were timely (11th year), and 23.2% were delayed. There was a bimodal distribution of the timing of the administration of the second dose, with peaks at the beginning of the 11th year and at the turn of the 11th and 12th year. Conclusions: The unexpected shape of the histograms suggests that ambiguous interpretations of the vaccination schedule may be one of the causes of vaccination delays.
Full article
(This article belongs to the Section Human Vaccines and Public Health)
►▼
Show Figures

Figure 1
Open AccessSystematic Review
Co-Administration of BNT162b2 COVID-19 and Influenza Vaccines in Adults: A Global Systematic Review
by
Constantina Boikos, Kassandra Schaible, Solange Nunez-Gonzalez, Verna Welch, Tianyan Hu, Moe Hein Kyaw, Laura Elizabeth Choi, Joanna Kamar, Henry Goebe and John McLaughlin
Vaccines 2025, 13(4), 381; https://doi.org/10.3390/vaccines13040381 - 2 Apr 2025
Abstract
Background/Objectives: Co-administration of BNT162b2 with licensed seasonal influenza vaccines (SIVs) is recommended by health authorities. We provide a comprehensive summary of the data supporting this practice in adults. Methods: This systematic review consolidates available evidence on the prevalence, safety, immunogenicity, efficacy, and effectiveness
[...] Read more.
Background/Objectives: Co-administration of BNT162b2 with licensed seasonal influenza vaccines (SIVs) is recommended by health authorities. We provide a comprehensive summary of the data supporting this practice in adults. Methods: This systematic review consolidates available evidence on the prevalence, safety, immunogenicity, efficacy, and effectiveness of co-administering BNT162b2 and SIVs. Searches were conducted for English studies in adults ≥ 18 years of age between January 2021 and August 2024, with no geographic restriction. Study quality was assessed using Cochrane RoB 2.0 and the Newcastle–Ottawa Scale. Results: Twenty studies (15 observational and 5 clinical trials) were included, mainly conducted in seven countries in Europe and North America. Eight observational studies reported prevalence, twelve reported safety/reactogenicity, six reported immunogenicity, and three evaluated efficacy/effectiveness. Reported co-administration of BNT162b2 vaccines with SIVs increased over time. Of persons receiving BNT162b2, the proportion that reported co-administered SIVs increased from 2.7% in 2021 to 34.1% in 2023. Although variability in outcomes was observed, no consistent pattern indicating a negative impact on immunogenicity from same-day co-administration was identified. Effectiveness was not observed to change when BNT162B2 was co-administered with SIVs. The incidence of systemic and local adverse events was comparable between individuals receiving the vaccines separately and those receiving them co-administered. Conclusions: The findings from this review indicate that the co-administration of BNT162B2 with SIVs is both safe and effective. This highlights the value of co-administration, which could enhance vaccine uptake by streamlining immunization protocols and reducing health visits.
Full article
(This article belongs to the Special Issue Vaccines and Vaccine Preventable Diseases)
►▼
Show Figures

Figure 1
Open AccessArticle
Microneedle Delivery of Heterologous Microparticulate COVID-19 Vaccine Induces Cross Strain Specific Antibody Levels in Mice
by
Tanisha Manoj Arte, Smital Rajan Patil, Emmanuel Adediran, Revanth Singh, Priyal Bagwe, Mahek Anil Gulani, Dedeepya Pasupuleti, Amarae Ferguson, Susu M. Zughaier and Martin J. D’Souza
Vaccines 2025, 13(4), 380; https://doi.org/10.3390/vaccines13040380 - 1 Apr 2025
Abstract
Background: In recent years, the COVID-19 pandemic has significantly impacted global health, largely driven by the emergence of various genetic mutations within the SARS-CoV-2 virus. Although the pandemic phase has passed, the full extent of the virus’s evolutionary trajectory remains uncertain, highlighting the
[...] Read more.
Background: In recent years, the COVID-19 pandemic has significantly impacted global health, largely driven by the emergence of various genetic mutations within the SARS-CoV-2 virus. Although the pandemic phase has passed, the full extent of the virus’s evolutionary trajectory remains uncertain, highlighting the need for continued research in vaccine development to establish a cross-reactive approach that can effectively address different variants. This proof-of-concept study aimed to assess the effectiveness of microparticulate vaccine delivery through the minimally invasive microneedle route of administration, using a heterologous prime–booster strategy against the SARS-CoV-2 virus. Method: This strategy uses the whole inactivated virus of the Delta variant for the prime dose and the whole inactivated virus of the Omicron variant for the booster dose, with alum as an adjuvant. The formulation of microparticles involves encapsulating the antigens in poly lactic-co-glycolic acid (PLGA) polymer, which provides sustained release and enhances immunogenicity while protecting the antigen. Microparticles were tested for in vitro assays, and characterization included particle size, zeta potential, and encapsulation efficacy. Furthermore, serum was collected post-administration of the vaccine in mice and was tested for antibody levels. Result: In vitro assays confirmed the non-cytotoxicity and the ability of microparticles to activate the immune response of the vaccine particles. Administering this microparticulate vaccine via microneedles has proven effective for delivering vaccines through the skin. We also observed significantly higher antigen-specific antibody levels and cross-reactivity in the strains. Conclusions: Our adjuvanted microparticulate-based heterologous prime–booster vaccine strategy showed cross-reactivity among the strains and was successfully delivered using microneedles.
Full article
(This article belongs to the Special Issue Advances in Vaccine Adjuvants)
►▼
Show Figures
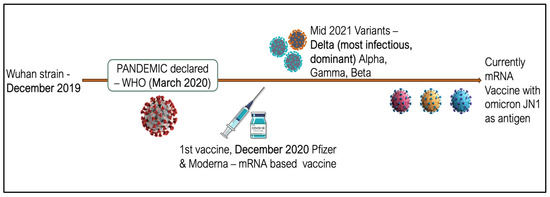
Figure 1
Open AccessArticle
Safety, Tolerability, and Immunogenicity of aH5N1 Vaccine in Adults with and Without Underlying Immunosuppressive Conditions
by
Peter Malfertheiner, Eve Versage, Esther Van Twuijver, Giuliano Rizzardini and Matthew Hohenboken
Vaccines 2025, 13(4), 379; https://doi.org/10.3390/vaccines13040379 - 1 Apr 2025
Abstract
►▼
Show Figures
Background: Pandemic influenza may cause substantial morbidity and mortality, especially in older adults and those with immunosuppressive conditions. Methods: In this phase 3, stratified, randomized, controlled, observer-blind, multicenter trial, we evaluated the safety, tolerability, and immunogenicity of an adjuvanted H5N1 vaccine
[...] Read more.
Background: Pandemic influenza may cause substantial morbidity and mortality, especially in older adults and those with immunosuppressive conditions. Methods: In this phase 3, stratified, randomized, controlled, observer-blind, multicenter trial, we evaluated the safety, tolerability, and immunogenicity of an adjuvanted H5N1 vaccine (aH5N1) vs. active control (MF59-adjuvanted trivalent seasonal inactivated influenza vaccine [aTIV]) in 539 adults aged 18–60 and ≥61 years. Participants were further stratified into subgroups that were healthy (18–60 years, n = 91; ≥61 years, n = 89) or had prespecified immunosuppressive conditions (18–60 years, n = 180; ≥61 years, n = 179). Antibody responses were measured with microneutralization and single radial hemolysis (SRH) assays. Results: aH5N1 increased antibody responses in healthy persons and those with immunosuppressive conditions in both age groups, with SRH geometric mean ratios (GMRs) > 2.5 and >2.0 in participants aged 18–60 and ≥61 years, respectively, meeting former Committee for Medicinal Products for Human Use (CHMP) criteria. Responses measured with the microneutralization and SRH assays were consistent with previous studies of aH5N1. Conclusions: The aH5N1 vaccine had a clinically acceptable safety and tolerability profile with an AE profile comparable to that observed in previous aH5N1 studies. These findings support the viability of aH5N1 as a pre-pandemic influenza vaccine for the immunization of at-risk individuals when an antigenically matched pandemic influenza vaccine is not yet available.
Full article

Figure 1
Open AccessReview
Plant-Derived Immunomodulatory Nanoadjuvants for Cancer Vaccines: Current Status and Future Opportunities
by
Yimin Jia, Hui Zhu, Xinyu Cai, Cun Sun, Yan Ye, Dingyi Cai, Shuaifei Yang, Jingjing Cheng, Jining Gao, Yun Yang, Hao Zeng, Quanming Zou, Jieping Li, Hongwu Sun and Wenxiu Wang
Vaccines 2025, 13(4), 378; https://doi.org/10.3390/vaccines13040378 - 31 Mar 2025
Abstract
Cancer is a major cause of death worldwide, and vaccine administration is an effective way to stimulate immune responses in patients and to achieve preventive and therapeutic effects. Few vaccines have been used in clinical settings because they have poor immunogenicity, and it
[...] Read more.
Cancer is a major cause of death worldwide, and vaccine administration is an effective way to stimulate immune responses in patients and to achieve preventive and therapeutic effects. Few vaccines have been used in clinical settings because they have poor immunogenicity, and it is difficult to induce a robust immune response in patients. An adjuvant is an important component of a vaccine that can enhance the intensity, speed, and duration of immune responses. The achievements of adjuvants in the production of stable, safe, and immunogenic tumor vaccines have aroused the enthusiasm of researchers. Recent results have suggested that plant-derived adjuvants have unique advantages, such as greatly improving immune responses to cancer vaccines and promoting humoral and cellular immunity with good biocompatibility and biodegradability. When these adjuvants are used in combination with vaccines, they can not only activate the immune response in vivo but can also promote cytokine secretion and accelerate dendritic cell maturation. This review focused on the application progress of plant adjuvants, including saponins, polysaccharides, flavonoids, and plant virus-like particles, and their combination with nano-delivery systems in cancer vaccines. At the same time, we have also discussed the immunomodulatory mechanisms of these adjuvants and their prospects for improving vaccine efficacy in the treatment of cancer in the future. These promising plant adjuvants may provide prospects and a research basis for the development of tumor vaccines.
Full article
(This article belongs to the Special Issue Research in Vaccine Adjuvants: Innovations and Challenges)
►▼
Show Figures
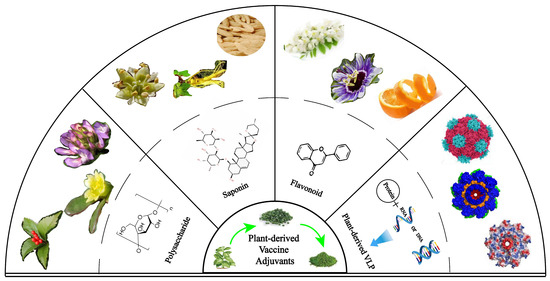
Figure 1
Open AccessSystematic Review
Immunization Coverage, Equity, and Access for Children with Disabilities: A Scoping Review of Challenges, Strategies, and Lessons Learned to Reduce the Number of Zero-Dose Children
by
Godfrey Musuka, Diego F. Cuadros, F. DeWolfe Miller, Zindoga Mukandavire, Tapiwa Dhliwayo, Patrick Gad Iradukunda, Oscar Mano and Tafadzwa Dzinamarira
Vaccines 2025, 13(4), 377; https://doi.org/10.3390/vaccines13040377 - 31 Mar 2025
Abstract
Background: Children with disabilities, particularly in low- and middle-income countries (LMICs), face heightened risks of vaccine-preventable diseases due to a range of systemic and social barriers. Although immunization is a fundamental human right and a proven public health intervention, this vulnerable group
[...] Read more.
Background: Children with disabilities, particularly in low- and middle-income countries (LMICs), face heightened risks of vaccine-preventable diseases due to a range of systemic and social barriers. Although immunization is a fundamental human right and a proven public health intervention, this vulnerable group is often overlooked in policy and practice. Understanding the factors compromising vaccine equity for these children is critical to reducing zero-dose prevalence and improving health outcomes. Methods: This scoping review examined peer-reviewed, gray literature from 2010 to 2024. Searches were conducted in PubMed, Google Scholar, and relevant organizational reports (WHO, UNICEF). Studies addressing children with disabilities and focusing on immunization barriers, interventions, or lessons learned were selected. English-language publications were screened in title/abstract and full-text stages. Key data extracted included population, barriers, and immunization outcomes. Since this review focused on articles in English, this is a key limitation. Results were synthesized thematically to identify recurring patterns and to guide improved interventions and policies. Results: Twelve articles met the inclusion criteria. Key barriers identified were inadequate healthcare infrastructure, insufficient provider training, limited follow-up services in rural regions, societal stigma, and pervasive misconceptions around both disability and vaccines. Factors such as maternal education, logistical support for caregivers, and using low-sensory, inclusive vaccination settings were consistently linked with better outcomes. Effective strategies included mobile vaccination units, tailored interventions (e.g., distraction or sedation techniques), school-based immunization programs, and robust community engagement to address stigma. Lessons learned underscored the importance of flexible, individualized care plans and empowering families through transparent communication. Conclusions: Children with disabilities continue to experience significant gaps in immunization coverage, driven by intersecting barriers at the individual, health system, and societal levels. Scaling tailored interventions, inclusive policies, strengthened infrastructure, and ongoing research can help ensure these children receive equitable access to life-saving vaccinations.
Full article
(This article belongs to the Special Issue 50 Years of Immunization—Steps Forward)
►▼
Show Figures

Figure 1
Open AccessArticle
Maternal Vaccination and Neonatal Feeding Strategies Among Polish Women
by
Jolanta Lis-Kuberka and Magdalena Orczyk-Pawiłowicz
Vaccines 2025, 13(4), 376; https://doi.org/10.3390/vaccines13040376 - 31 Mar 2025
Abstract
Background/Objectives: Maternal vaccination and breastfeeding are important aspects of public health that should be recommended by medical staff caring for pregnant and postpartum women. We aimed to analyze factors affecting women’s likelihood of dual vaccination during pregnancy and their infant feeding strategies.
[...] Read more.
Background/Objectives: Maternal vaccination and breastfeeding are important aspects of public health that should be recommended by medical staff caring for pregnant and postpartum women. We aimed to analyze factors affecting women’s likelihood of dual vaccination during pregnancy and their infant feeding strategies. Methods: A cross-sectional study was conducted with 953 Polish mothers. An online questionnaire was used and included questions on sociodemographic and obstetric variables, women’s attitudes towards COVID-19 and influenza vaccination, and breastfeeding practices. Results: COVID-19 vaccination was reported by 66.0%, influenza vaccination by 18.2%, and dual vaccination by 15.6% of Polish mothers. Increasing willingness to receive vaccines was significantly associated with older maternal age, lower BMI, living in urban areas with >100,000 residents, and high levels of knowledge regarding vaccination. No significant association between dual vaccination and neonatal feeding strategy was detected. The group of exclusively breastfeeding mothers, in comparison to formula- and mixed-feeding women, was characterized by having lower pre-pregnancy BMI and previous maternal experience. Conclusions: Rates of vaccination against seasonal influenza and dual (influenza and COVID-19) vaccination remain low among Polish mothers. The promotion of antenatal vaccination and reliable information about short- and long-term advantages related to breastfeeding are crucial to perinatal health care for the mother–infant dyad. Young, primiparous women who are overweight or obese should be targets of preventive programs focused on the health of the mother–infant dyad.
Full article
(This article belongs to the Special Issue Vaccines and Prevention of Infections in Early Life)
►▼
Show Figures

Figure 1
Open AccessArticle
Impact of Schistosoma mansoni Infection on the Gut Microbiome and Hepatitis B Vaccine Immune Response in Fishing Communities of Lake Victoria, Uganda
by
Yan Wang, Ariana K. Waters, Geofrey Basalirwa, Ali Ssetaala, Juliet Mpendo, Annemarie Namuniina, Emily Keneema, David Kiiza, Jacqueline Kyosiimire-Lugemwa, Yunia Mayanja, Brenda Okech and Sylvia Kiwuwa-Muyingo
Vaccines 2025, 13(4), 375; https://doi.org/10.3390/vaccines13040375 - 31 Mar 2025
Abstract
►▼
Show Figures
Objective: Schistosoma mansoni (S. mansoni) infection is endemic in Ugandan fishing communities. We investigated its potential impact on Hepatitis B (Hep B) vaccine responses and its role in mediating the association between the gut microbiome and long-term effectiveness of the vaccine.
[...] Read more.
Objective: Schistosoma mansoni (S. mansoni) infection is endemic in Ugandan fishing communities. We investigated its potential impact on Hepatitis B (Hep B) vaccine responses and its role in mediating the association between the gut microbiome and long-term effectiveness of the vaccine. Methods: Participants were tested for S. mansoni infections at baseline and received the Hep B vaccine at baseline, month 1, and month 6. Those with infections were treated. Stool samples were collected at baseline and analyzed using 16S rRNA sequencing. The Wilcoxon rank-sum test was used to compare alpha diversity between groups. A linear regression model was applied to estimate the association between one-year Hep B vaccine responses and the baseline gut microbiome by infection status, adjusting for age and sex. Results: A total of 107 participants were included (44 from the fishing community and 63 from the Kampala community). There was no significant difference in microbiome composition by location or infection status at baseline or discharge. In the linear regression analysis, S. mansoni infection (β = 1.24, p = 0.025) and a higher alpha diversity (β = 0.001, p = 0.07) were associated with higher Hep B vaccine responses, while older age was associated with a lower Hep B vaccine response (β = −0.06, p = 0.0013). Conclusions: S. mansoni infection status before vaccination may modify the association between the gut microbiome and Hep B vaccine response. Potential interventions could focus on infection control as well as improving microbiome richness before implementing vaccine programs in fishing communities.
Full article
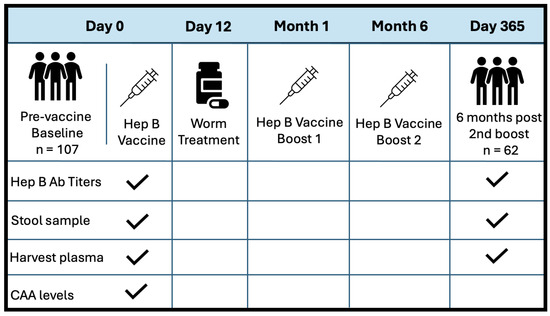
Figure 1
Open AccessArticle
An Mpox Multi-Antigen-Tandem Bivalent mRNA Candidate Vaccine Effectively Protects Mice Against the Vaccinia Virus
by
Jun Zuo, Jiayu Wu, Zhen Zhang, Jinrong Long, Changxiao Yu, Yuqin Liao, Hongsheng Zhang and Jing Yang
Vaccines 2025, 13(4), 374; https://doi.org/10.3390/vaccines13040374 - 31 Mar 2025
Abstract
►▼
Show Figures
Background: Since the outbreak of mpox in 2022, the disease has spread rapidly worldwide and garnered significant public attention. Vaccination is regarded as an effective measure to prevent the spread of mpox. The success of the COVID-19 mRNA vaccine demonstrates that mRNA-based vaccines
[...] Read more.
Background: Since the outbreak of mpox in 2022, the disease has spread rapidly worldwide and garnered significant public attention. Vaccination is regarded as an effective measure to prevent the spread of mpox. The success of the COVID-19 mRNA vaccine demonstrates that mRNA-based vaccines represent a rapid and multifunctional platform with considerable potential, and are expected to be a strategy to address mpox spread. Methods: In this study, we screened an mpox multi-antigen-tandem bivalent mRNA vaccine candidate: a lipid nanoparticle-encapsulated mRNA-1017 and mRNA-1995 (mRNA-3012-LNP). We then evaluated the immunogenicity of the mpox virus (MPXV) bivalent mRNA vaccine candidate and its protective efficacy against the vaccinia virus (VACV) in a mouse model. Results: Mice vaccinated with two doses of the mRNA-3012-LNP vaccine exhibited robust binding antibody responses and MPXV-specific Th-1-biased cellular immune responses in vivo. Notably, the boosted immunized mice generated potent neutralizing antibodies against the VACV, effectively protecting them from viral challenge. Additionally, serum transfer protection experiments indicated that serum from mice inoculated with mRNA-3012-LNP was effective in protecting nude mice from VACV challenge. Conclusions: Our results suggest that the mpox bivalent mRNA candidate vaccine mRNA-3012-LNP induces strong immunogenicity and has the potential to serve as a safe and effective vaccine candidate against mpox epidemics.
Full article
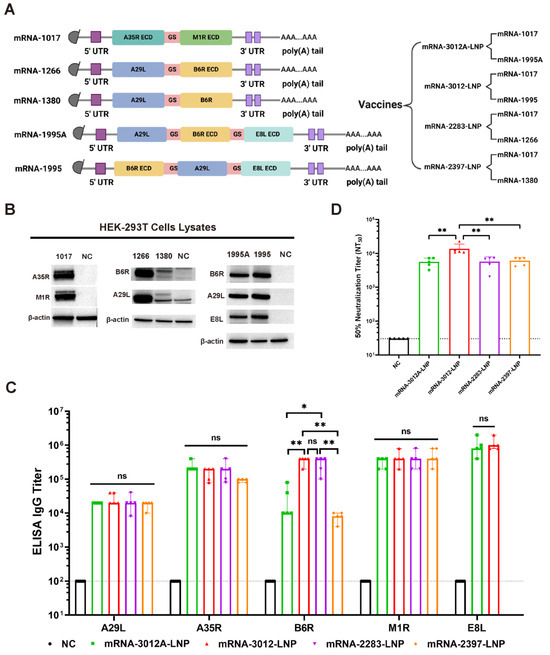
Figure 1
Open AccessArticle
Regional Disparities in HPV Vaccination Coverage Among Girls Aged 9 to 14 Years in Togo: Lessons Learned from the Recent Supplementary Immunization Activities
by
Dadja Essoya Landoh, Issifou Yaya, Amevegbe Boko, Kodjovi Adjeoda, Yaovi Temfan Toke, Adidja Amani, Yerima Mouhoudine, Ado Mpia Bwaka, Nsiari-Mueyi Joseph Biey, Charles Shey Wiysonge, Franck Fortune Roland Mboussou, Hèzouwè Looky-Djobo, Tsidi Agbeko Tamekloe, Toyi Nyulelen Mangbassim, Tchasso Kenao, Amadou Bailo Diallo, Fatoumata Binta Tidiane Diallo, Benido Impouma, Ann Lindstrand, Marin Kokou Wotobe and Didier Koumavi Ekoueviadd
Show full author list
remove
Hide full author list
Vaccines 2025, 13(4), 373; https://doi.org/10.3390/vaccines13040373 - 31 Mar 2025
Abstract
Background/Objectives: Human papillomavirus (HPV) vaccination is a critical intervention to prevent cervical cancer, especially in settings where screening is limited. In Togo, cervical cancer is the second most common cancer in women. Togo conducted an HPV vaccination campaign for girls aged 9–14 from
[...] Read more.
Background/Objectives: Human papillomavirus (HPV) vaccination is a critical intervention to prevent cervical cancer, especially in settings where screening is limited. In Togo, cervical cancer is the second most common cancer in women. Togo conducted an HPV vaccination campaign for girls aged 9–14 from 27 November to 1 December 2023, followed by introduction of the vaccine into routine immunization. This study aimed to assess regional disparities in vaccination coverage during this campaign. Methods: A cross-sectional study was conducted using data from the nationwide HPV vaccination campaign. The target population included girls aged 9–14, following school and community-based enumeration. The campaign employed school-based, health facility-based, and community-based vaccination strategies. Data were collected via multiple tools, and monitoring was carried out through daily reports and digital tracking. Results: Out of the estimated 654,402 eligible girls, 304,457 (46.5%) were vaccinated. Vaccine coverage varied significantly by region, ranging from 76% in Kara to 15% in Grand Lomé. In-school settings accounted for 91.3% of vaccinations, with the fixed strategy covering 55.4%. In total, 11 districts exceeded 80% vaccine coverage, while 15 districts had <50%. The highest rate of adverse events following immunization was observed in the Maritime region, primarily involving minor symptoms. Conclusion: Although progress was made in HPV vaccination coverage in Togo, regional disparities highlight the need for targeted interventions. Strategies such as expanding vaccine access, enhancing awareness campaigns, and integrating HPV vaccination into routine immunization could improve coverage. Addressing logistical and cultural barriers is also crucial for equitable vaccination, aiming to achieve international benchmarks and reduce HPV-related disease burdens. Further research should explore qualitative factors influencing vaccine acceptance.
Full article
(This article belongs to the Special Issue 50 Years of Immunization—Steps Forward)
►▼
Show Figures

Figure 1
Open AccessArticle
Fc-Modified Antibody in Hospitalized Severe COVID-19 Patients
by
Felipe Dal-Pizzol, Suzana Margareth Lobo, Christopher Lucasti, Adam Abdul Hakeem Baidoo, Huo Su, Zhanghua Lan and Liangzhi Xie
Vaccines 2025, 13(4), 372; https://doi.org/10.3390/vaccines13040372 - 31 Mar 2025
Abstract
Background: Hospitalized patients with severe COVID-19 are at high risk of clinical deterioration. Methods: A global, randomized, double-blinded, and placebo-controlled phase II trial that investigated the clinical efficacy of SCTA01, an Fc-modified monoclonal antibody, in patients hospitalized with severe COVID-19 during the Delta
[...] Read more.
Background: Hospitalized patients with severe COVID-19 are at high risk of clinical deterioration. Methods: A global, randomized, double-blinded, and placebo-controlled phase II trial that investigated the clinical efficacy of SCTA01, an Fc-modified monoclonal antibody, in patients hospitalized with severe COVID-19 during the Delta variant wave was performed. The primary outcome was time to clinical improvement up to Day 29. Secondary outcomes measured the all-cause mortality rate up to Day 29, time to SARS-CoV-2 RNA negativity up to Day 29, and the number of antibody-dependent enhancements. Results: From 27 March 2021, to 11 February 2022, 102 hospitalized adults with severe COVID-19 received a single intravenous infusion of SCTA01 15 mg/kg or 50 mg/kg or placebo in a 1:1:1 ratio. The median time to clinical improvement in the SCTA01 group was numerically shorter than that in the placebo group; however, the between group difference was statistically non-significant (SCTA01 15 mg/kg vs. placebo, HR 0.99, 95% CI 0.55–1.77, p = 0.742; SCTA01 50 mg/kg vs. placebo, HR 1.07, 95% CI 0.61–1.88, p = 0.095). The median time to achieve a negative SARS-CoV-2 status was shorter in the SCTA01 15 mg/kg group (14.0 days vs. 27.0 days) but not in the SCTA01 50 mg/kg group (28.0 days vs. 27.0 days) compared to the placebo group. Adverse events were comparable across all groups, and no treatment-related serious adverse event or antibody-dependent enhancement was reported. Conclusions: The Fc-modified antibody was safe but lacked significant clinical efficacy in vivo, likely due to the SARS-CoV-2 viral mutation.
Full article
(This article belongs to the Special Issue Review Special Issue Series—Measuring Neutralizing Antibody Responses Against SARS-CoV-2 and Emerging Infectious Diseases)
►▼
Show Figures
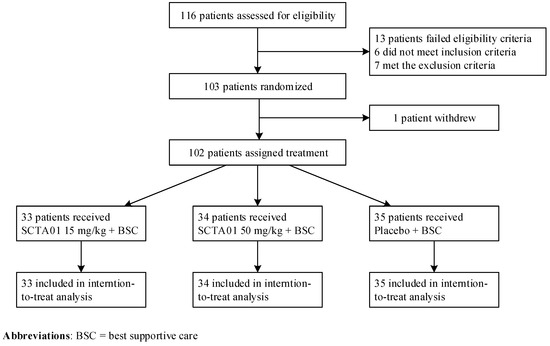
Figure 1

Journal Menu
► ▼ Journal Menu-
- Vaccines Home
- Aims & Scope
- Editorial Board
- Reviewer Board
- Topical Advisory Panel
- Instructions for Authors
- Special Issues
- Topics
- Sections & Collections
- Article Processing Charge
- Indexing & Archiving
- Editor’s Choice Articles
- Most Cited & Viewed
- Journal Statistics
- Journal History
- Journal Awards
- Conferences
- Editorial Office
Journal Browser
► ▼ Journal BrowserHighly Accessed Articles
Latest Books
E-Mail Alert
News
2 April 2025
MDPI INSIGHTS: The CEO's Letter #21 - Annual Report, Swiss Consortium, IWD, ICARS, Serbia
MDPI INSIGHTS: The CEO's Letter #21 - Annual Report, Swiss Consortium, IWD, ICARS, Serbia
1 April 2025
Meet us at the 11th Congress of the European Academy of Neurology, 21–24 June 2025, Helsinki, Finland
Meet us at the 11th Congress of the European Academy of Neurology, 21–24 June 2025, Helsinki, Finland

Topics
Topic in
Animals, Arthropoda, Insects, Vaccines, Veterinary Sciences, Pathogens
Ticks and Tick-Borne Pathogens: 2nd Edition
Topic Editors: Alina Rodriguez-Mallon, Alejandro Cabezas-CruzDeadline: 31 March 2026

Conferences
Special Issues
Special Issue in
Vaccines
Research on Immune Response and Vaccines: 2nd Edition
Guest Editor: James GallowayDeadline: 30 April 2025
Special Issue in
Vaccines
Current Development of Vaccines for Respiratory Viral Infection
Guest Editor: Ting ShiDeadline: 30 April 2025
Special Issue in
Vaccines
Advances in Vaccine Adjuvants
Guest Editors: Devyani Joshi, Sanjeev KumarDeadline: 30 April 2025
Special Issue in
Vaccines
HPV Vaccination Coverage: Problems and Challenges
Guest Editors: Shillpa Naavaal, Zheng Quan Toh, Paul LicciardiDeadline: 30 April 2025
Topical Collections
Topical Collection in
Vaccines
COVID-19 Vaccines and Vaccination
Collection Editors: Ralph Tripp, Scott Anthony
Topical Collection in
Vaccines
Factors Associated with Vaccine Hesitancy
Collection Editor: Brian D. Poole
Topical Collection in
Vaccines
COVID-19 Vaccine Hesitancy: Correlates and Interventions
Collection Editors: Manoj Sharma, Kavita Batra













